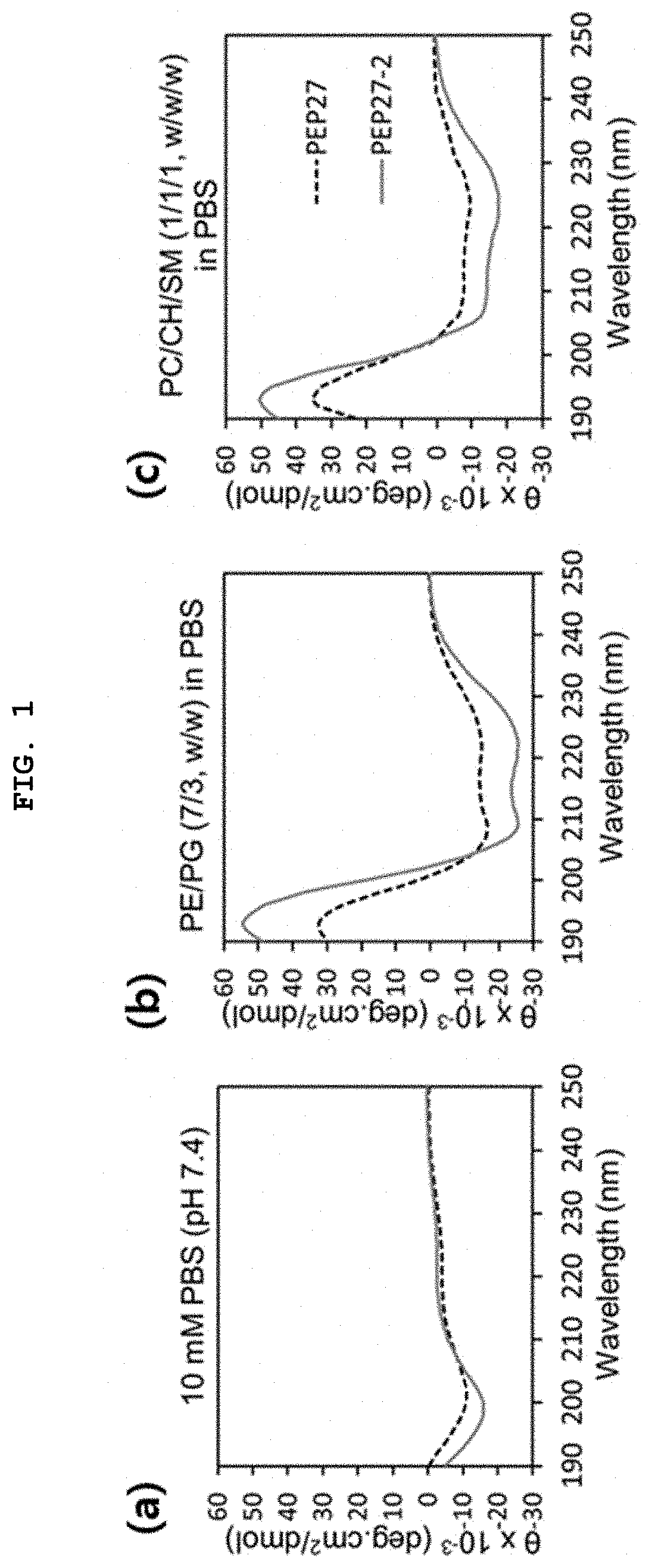
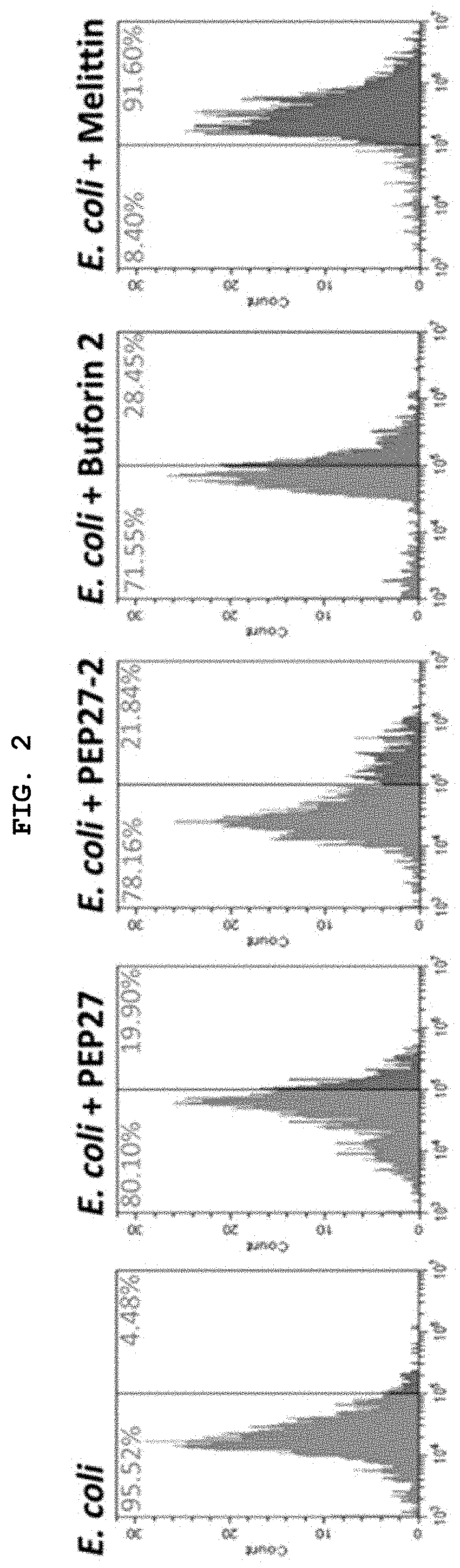
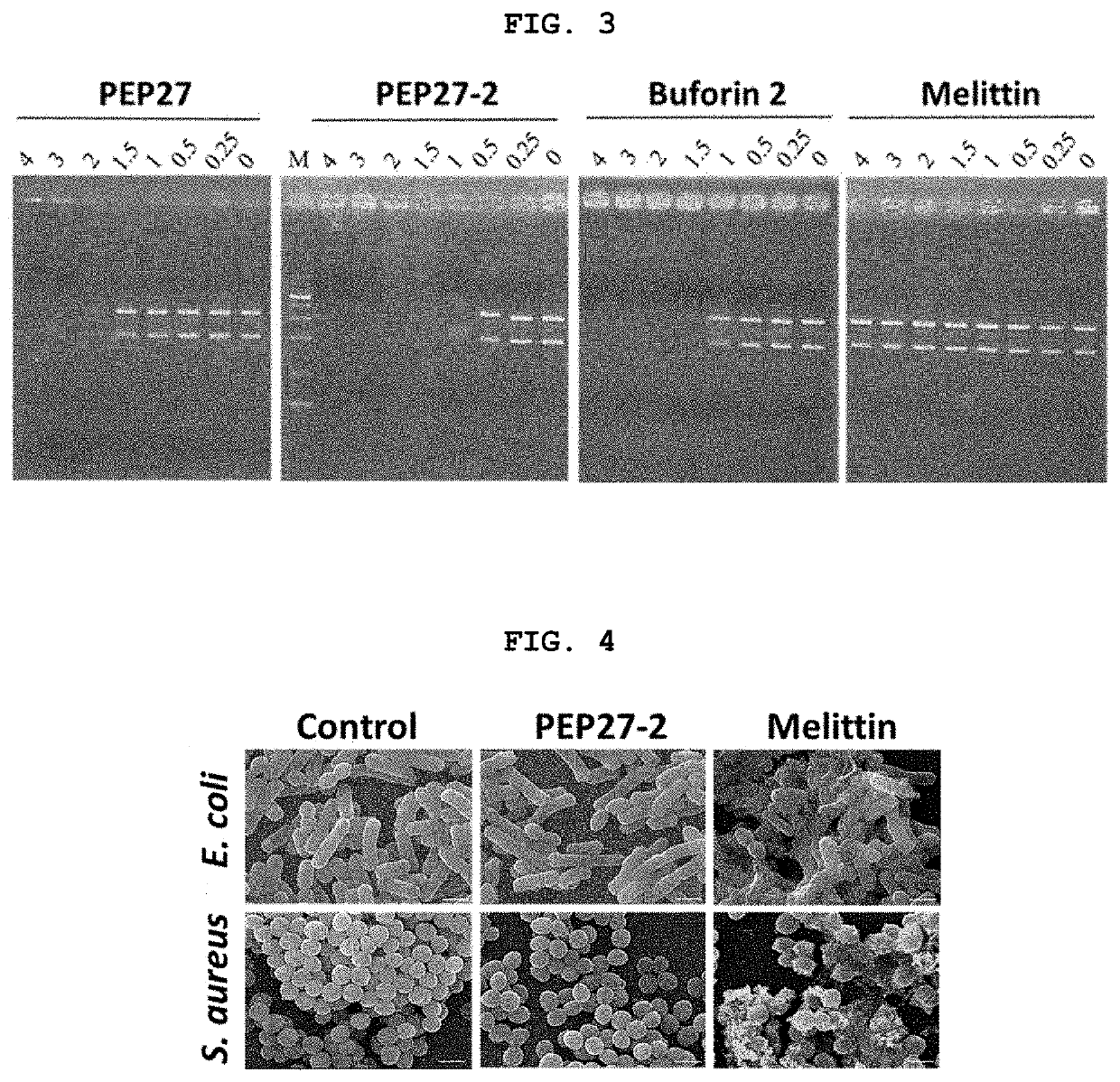
Novel peptide derived from pep27 peptide and uses thereof
a technology of pep27 and peptide, which is applied in the direction of peptides, peptides/protein ingredients, antibacterial agents, etc., can solve the problems of antibiotic treatment being less effective in clinical infections and bacteria's antibiotic resistance, and achieve excellent antimicrobial activity and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparative Preparation of Pharmaceutical Formulation
[0173]1-1. Preparation of Powder
TABLE 10ComponentWeight (mg)Peptide of the present20 mginventionLactose20 mg
[0174]After admixing the above components, the mixture was filled in an airtight cloth bag to prepare powder.
[0175]1-2. Preparation of Tablets
ComponentWeight (mg)Peptide of the present 10 mginventionCorn starch100 mgLactose100 mgMagnesium stearate 2 mg
[0176]After admixing the above components, the mixture was punched to form a tablet formulation according to the conventional tablet formation method.
[0177]1-3. Preparation of Capsules
TABLE 12ComponentWeight (mg)Peptide of the present 10 mginventionCrystalline cellulose 3 mgLactose14.8 mgMagnesium stearate 0.2 mg
[0178]After admixing the above components, the mixture was charged in a gelatin capsule to form a capsule formulation according to the conventional capsule preparation method.
[0179]1-4. Preparation of Liquid Formulation
TABLE 13ComponentWeight (mg)Peptide of the prese...
example 2
Preparative Preparation of Cosmetics
[0183]2-1. Emollient Lotion (Skin)
[0184]In order to prepare an antimicrobial emollient skin lotion containing the peptide of the present invention, the following components could be admixed as listed in Table 15 below to prepare the emollient skin lotion according to the conventional manufacturing method in the field of cosmetics.
TABLE 15ComponentContent (wt. %)Peptide of the present0.1 to 30invention1,3-butyleneglycol3.0Glycerin5.0Polyoxyethylene (60)0.2hydrogenated castor oilEthanol8.0Citric acid0.02Sodium citrate0.06PreservativeTrace amountFlavorTrace amountPurified waterTo 100
[0185]2-2. Nutritional Lotion (Lotion)
[0186]In order to prepare an antimicrobial nutritional lotion containing the peptide of the present invention, the following components could be admixed as listed in Table 16 below to prepare the nutritional lotion according to the conventional manufacturing method in the field of cosmetics.
TABLE 16ComponentContent (wt. %)Peptide of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
| time- | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com