Novel use of pyrrolopyridine derivatives for preventing or treating inflammatory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Inhibitory Activity of the Compounds According to the Present Invention on Various Kinases
[0109]Measuring the enzyme (kinase) selectivity and inhibitory activity of Compound 1 selected from among the example compound of the present invention was consigned to DiscoverX, and a scanMAX™ Kinase analysis panel was used to carry out the experiment. Here, the concentration of the drug used to treat the enzyme was 1 μM in DMSO; the percentage control (% control) was determined using the following method, and the results are shown in Table 2.
[(Example compound−positive control) / (negative control−positive control)×100]
[0110]Here, the positive control refers to a compound showing a percentage control of 0%, and the negative control indicates a percentage control of 100% with DMSO. Furthermore, regarding the enzyme selectivity of the present invention, if the percentage control for each enzyme was <41% (that is, less than 41%), the compound was judged to have activity with regard to such e...
example 2
n of Pro-Inflammatory Cytokine Inhibitory Activity of the Compounds According to the Present Invention
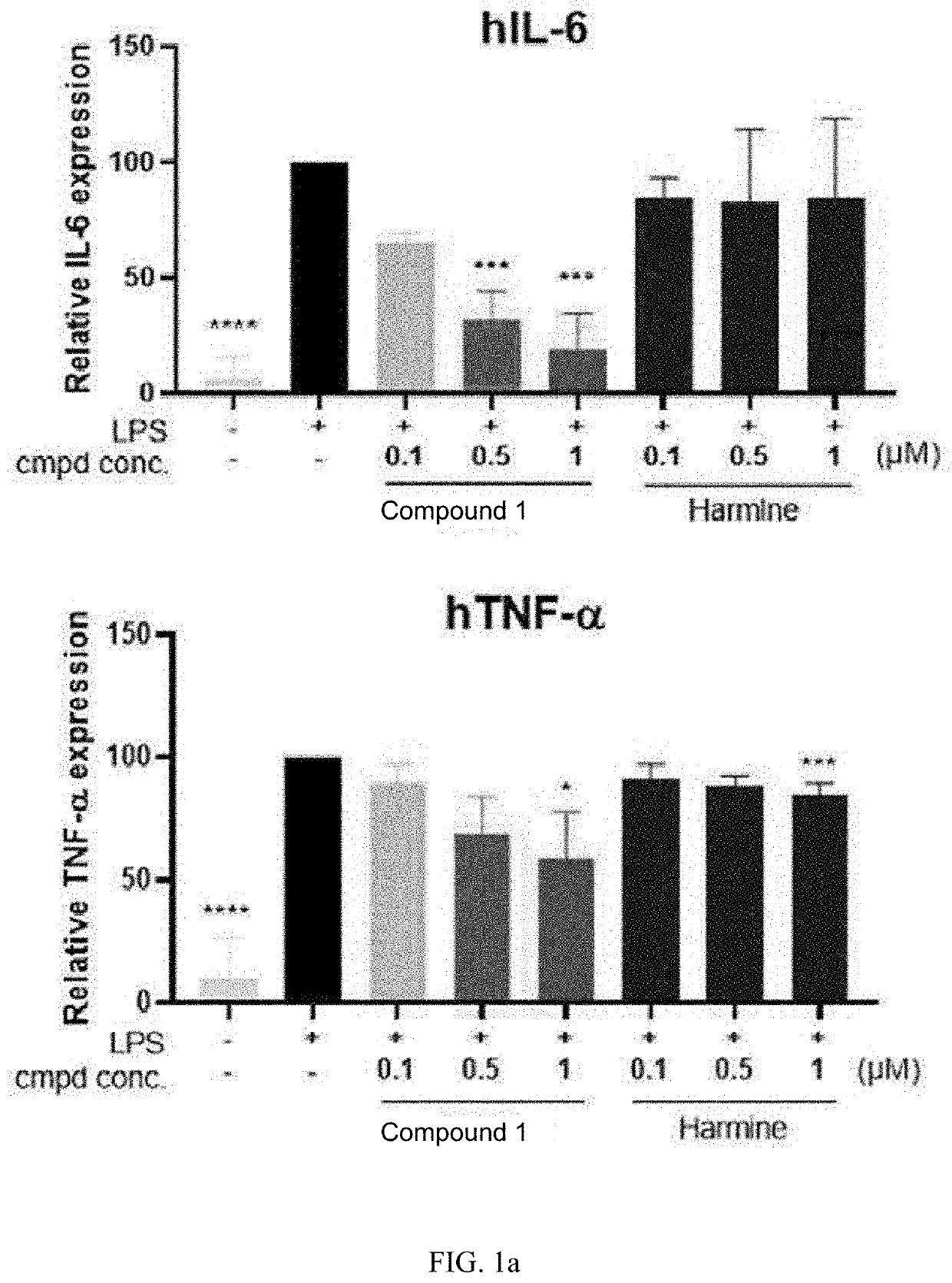
[0112]THP-1 cells were pre-treated with Compound 1 for 1 hour, and then treated with lipopolysaccharides (Lipopolysaccharides, LPS, 0.5 μg / ml), an inflammatory substance, for 24 hours to confirm the expression of the pro-inflammatory cytokines TNF-α and IL-6 by the THP-1 cells and the NF-κb signaling pathway associated with their expression.
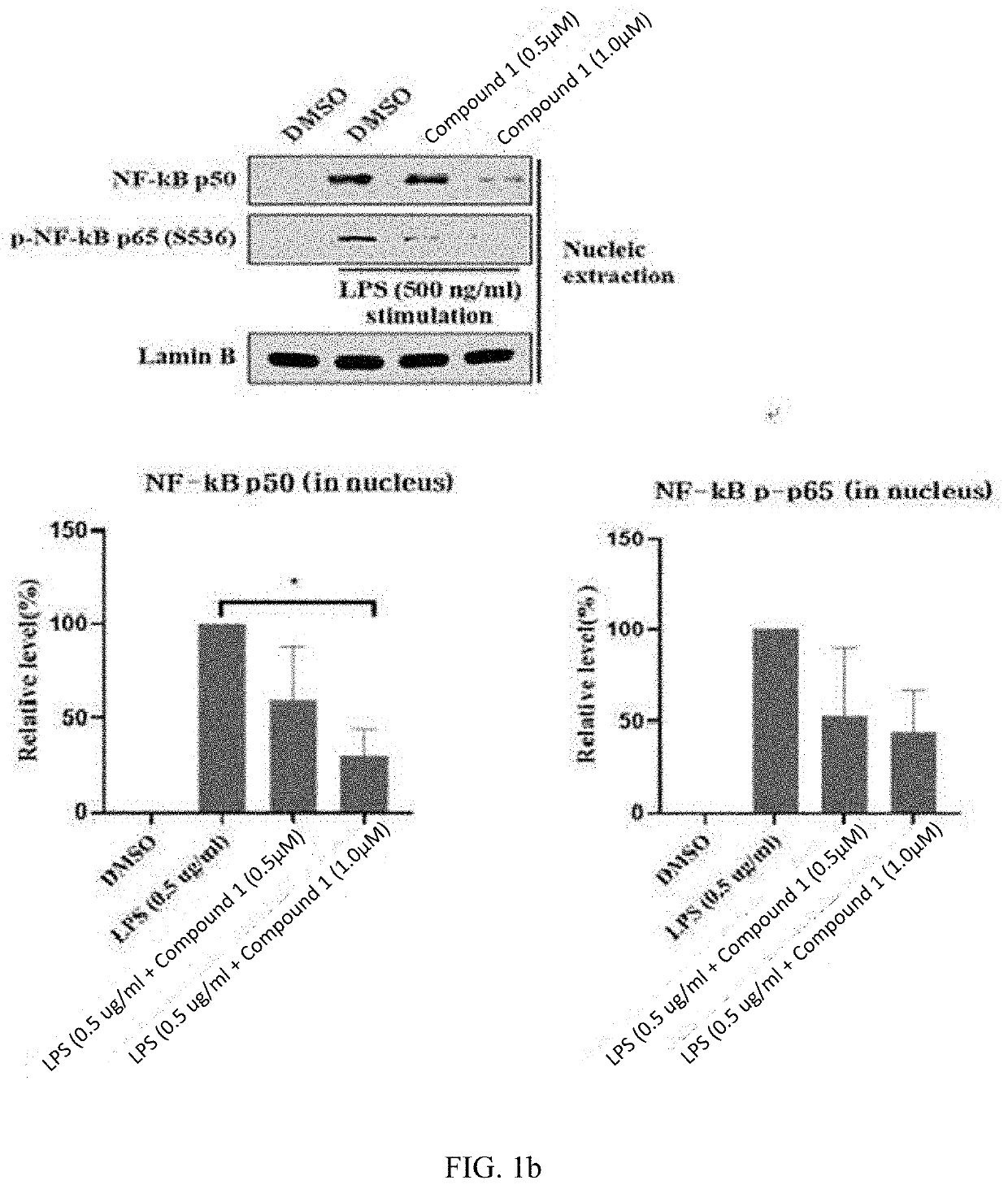
[0113]When the THP-1 cells were treated with LPS, the expression of the inflammation-inducing pro-inflammatory cytokines TNF-α and IL-6 increased, and their expression was effectively inhibited by treatment with Compound 1 (FIG. 1a); the expression of such pro-inflammatory cytokines is increased through activation of the NF-κb signaling pathway, and it can be seen that intranuclear movement of p50 and p65, which are associated with the NF-κb signaling pathway, is effectively inhibited by Compound 1 (FIG. 1b). These results confirm that expression...
example 3
n of the Regulation Activity of the Compounds According to the Present Invention Against Regulatory T Cell and Th17 Cell Differentiation
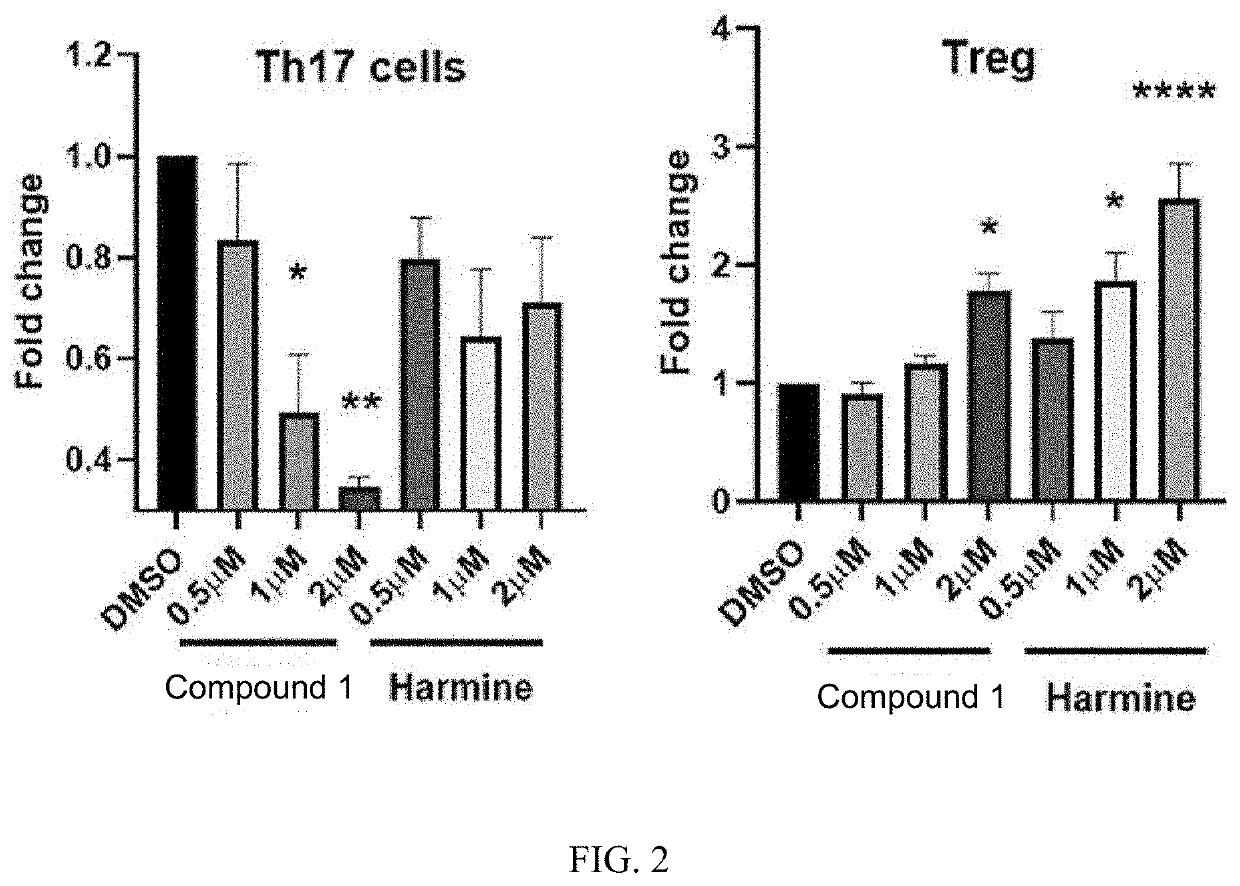
[0114]Using naive T cells in the mouse spleen, Compound 1 was sufficiently dissolved in DMSO for the differentiation conditions of regulatory T cells and Th17 cells to confirm differentiation. The experiment was conducted using Harmine as a control.
[0115]In the results, as shown in FIG. 2, when treated with Compound 1, it was found that differentiation of regulatory T cells (Treg) increased and differentiation of Th17 cells decreased, indicating potential for use in treatment of inflammatory diseases such as autoimmune disease.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com