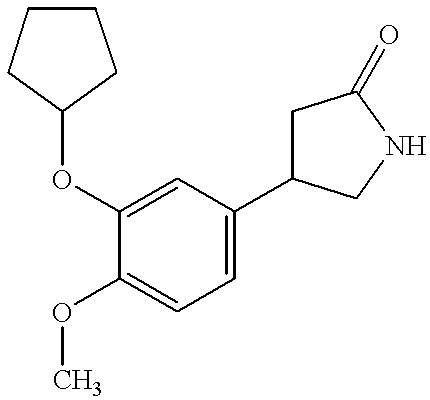
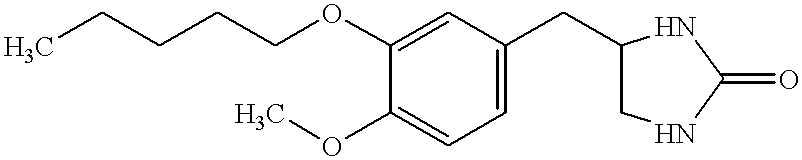
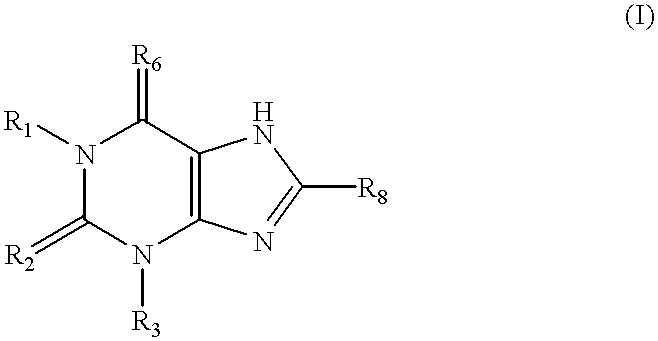
Trisubstituted thioxanthines
a technology of thioxanthines and substituted thioxanthines, which is applied in the field of trisubstituted thioxanthines, can solve the problems of narrow therapeutic index, increase in both force and rate of cardiac contractility, and limited number of structural classes of pde iv inhibitors, and achieve the effect of superior pde iv inhibitory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1,3-Diethyl-8-cyclopropyl-2,6-dithioxanthine
A. 1,3-Diethyl-8-cyclopropyl-2-thioxanthine
25.1 g (100 mM) of 5,6-diamino 1,3-diethyl-2-thiouracil HCl were dissolved in 400 ml of pyridine, 12.72 g (120 mM) of sodium carbonate added, and under cooling a solution of 10.77 ml (120 mM) of cyclopropane carbonyl chloride in 50 ml of dried ether added within 10 minutes. After 20 min the solvents were evaporated in vacuo. The residue was treated with 200 ml of water and about 50 ml were removed again in vacuo. The suspension was diluted with 100 ml of 2N aqueous sodium hydroxide (NaOH) and heated under reflux for 30 minutes. A further 80 ml was distilled off during this time. After cooling the solution was acidified with 5N aqueous hydrochloric acid (HCl) to pH 5.5; 200 ml of water was added and the resulting suspension filtered. The solid was collected and washed, redissolved in 200 ml of 1N NaOH, treated twice with 0.4 g of charcoal, filtered and acidified again to pH 4.5. The solid was colle...
example 2
3-(4-Chlorobenzyl)-1-ethyl-8-isopropyl-6-thioxanthine
A. 3-(4-Chlorobenzyl)-1-ethyl-8-isopropyl-xanthine
13.47 g (40 mM) of 6-amino-1-(4-chlorobenzyl)-5-isobutyrylaminouracil were dissolved in 130 ml of DMF, treated at 5.degree. C. with 4.57 g (40.8 mM) potassium t-butoxide and after dissolution, 3.28 ml (44 mM) of ethyl bromide added. After 3 hours, another 1.14 g of t-BuOK and 1.64 ml of ethyl bromide were added. After a further 1.5 hours, 1.64 ml of ethyl bromide was supplemented. After a total of 22 hours, the solution was neutralized with 1N HCl to pH 7 and the solvents evaporated in vacuo. The residue was taken up in dichloromethane-water and the organic phase collected giving 17.66 of crude 3-ethyl uracil, which was dissolved in 17.6 ml of 1N NaOH and heated under reflux for 1 hour. The solution was treated twice with 1 g of charcoal, filtered and neutralized with 5N HCl to pH 7. The solid was diluted with water, collected, and dried. The crude material was recrystallized from ...
example 3
1-(4-Chlorobenzyl)-3-ethyl-8-isopropyl-6-thioxanthine
A. 1-(4-Chlorobenzyl)-3-ethyl-8-isopropyl-xanthine
3.17 g (28.2 mM) of potassium t-butoxide (t-BuOK) were added to a solution of 6.11 g (27.5 mM) of 6-amino-1-ethyl-5-isobutyrylamino-uracil. At 0.degree. C., 4.90 g (30.4 mM) of 4-chlorobenzylchloride were added. After 3 hours at 0-5.degree. C., further 1.22 g t-BuOK and 2.45 g of 4-chloro-benzylchloride were added. After further 3 hours another 2.45 g of benzylchloride are supplemented. After 3 days, the solution was neutralized with 1N HCl and the solvents evaporated. The residue was suspended in water, the solid collected and washed. The crude intermediate amide was heated under reflux in 100 ml of 1N NaOH and 10 ml of 1-propanol. After 1 hour, the mixture was neutralized to pH 7 and extracted with chloroform. Crystallization from dichloromethane (mainly evaporated)-methanol gave 2.99 g (31.3%) of the title xanthine, mp 194-5.degree. C. The mother liquors gave 6.33 g of impure ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com