Device for determination of an analyte in a body fluid
a technology for body fluids and analytes, which is applied in the direction of analytical using chemical indicators, laboratory glassware, instruments, etc., can solve the problems of end users, contamination of meter, and limitations of the technology embodied in the products developed to da
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example a
ents
Reagent 1a
40 mg MBTH-S
80 mg DMAB
5 ml acetonitrile and 5 ml water
Stir until all solids are dissolved.
Reagent 2a
6 ml water
10 mg EDTA, disodium salt
200 mg PolyPep, low viscosity (Sigma)
0.668 g sodium citrate
0.523 g citric acid as a hematocrit adjuster
0.2 M Aconitic acid buffer
3% polyethylene glycol (PEG), as a separating agent
0.5% Polyquart, a binder
2.0 ml 6 wt % Gantrez AN-139 dissolved in water (GAF)
30 mg horseradish peroxidase, 100 units / mg, and 3.0 glucose oxidase, 2000 units / ml
Stir until dissolved.
example b
ents
Reagent 1b
20 ml water
420 mg citric acid (a buffering agent). Adjust the pH of the citric acid solution with NaOH to a value of 4.25.
16.7 mg EDTA
90 mg Gantrez S95 available from GAF
250 mg Crotein SPA
20,500 units glucose oxidase
16,200 units peroxidase
Reagent 2b
10 ml of a mixture of 3 parts by volume water and 7 parts by volume isopropyl alcohol
13 mg MBTH-S
40 mg ANS
Polyethersulfone Matrix
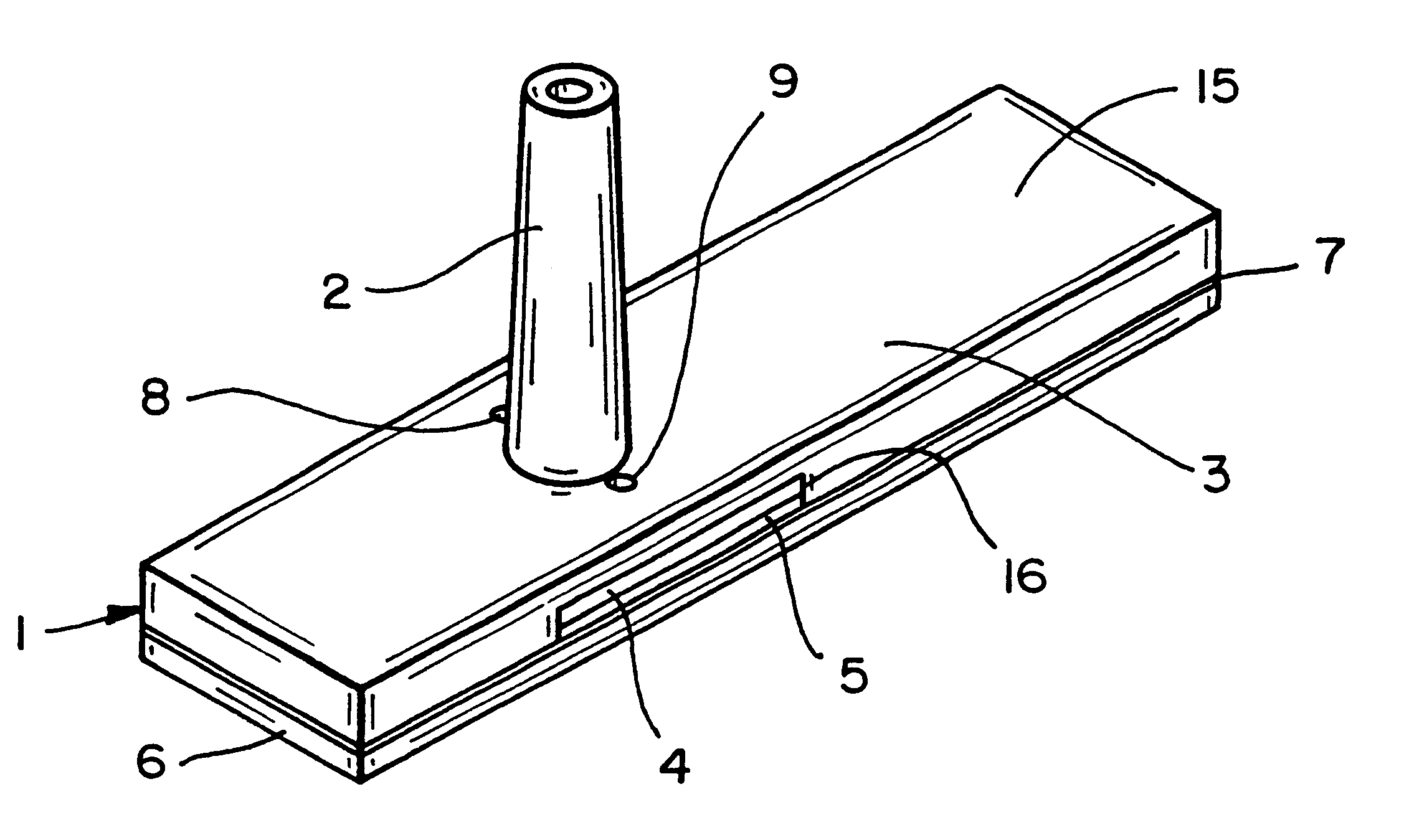
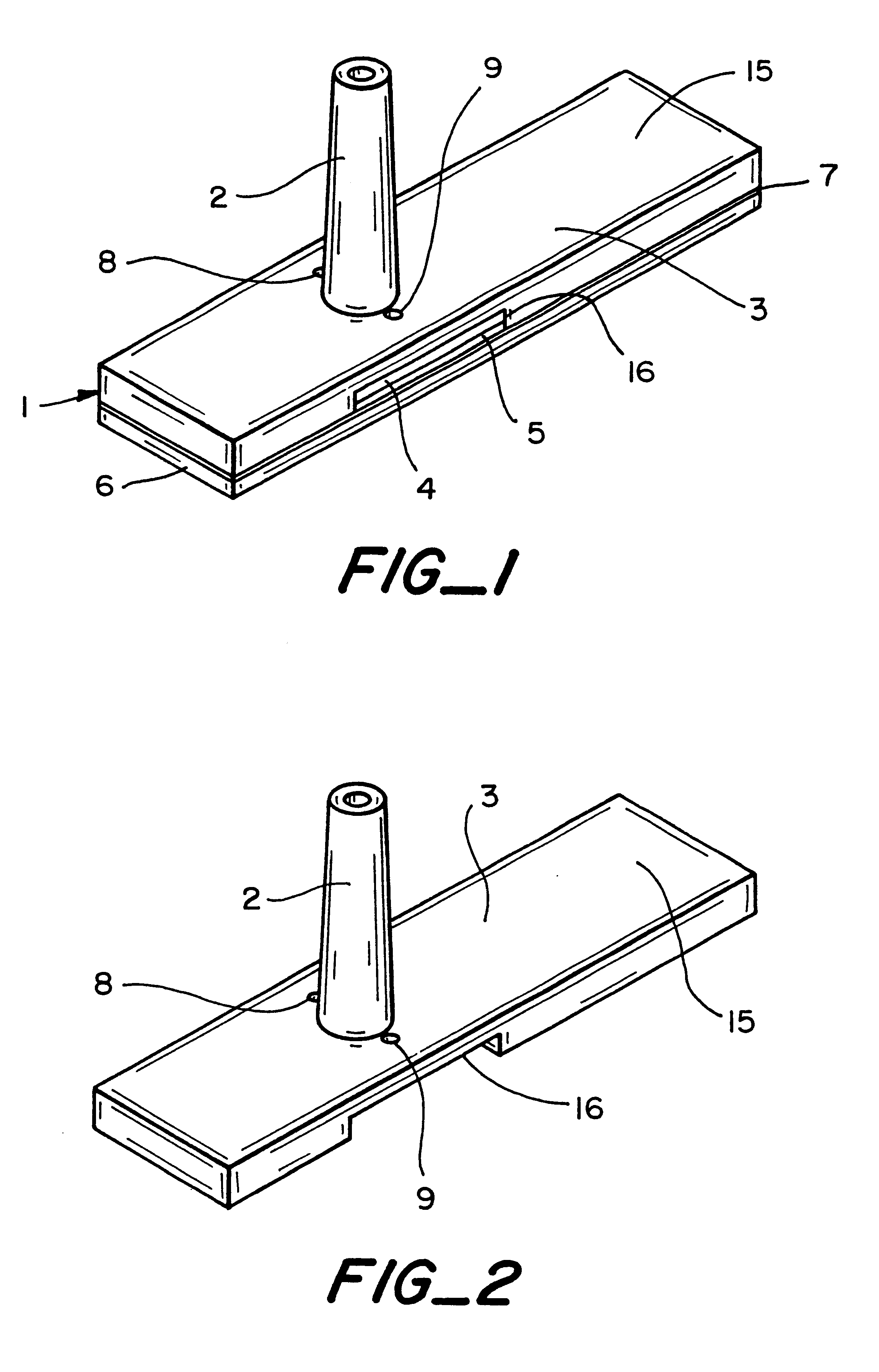
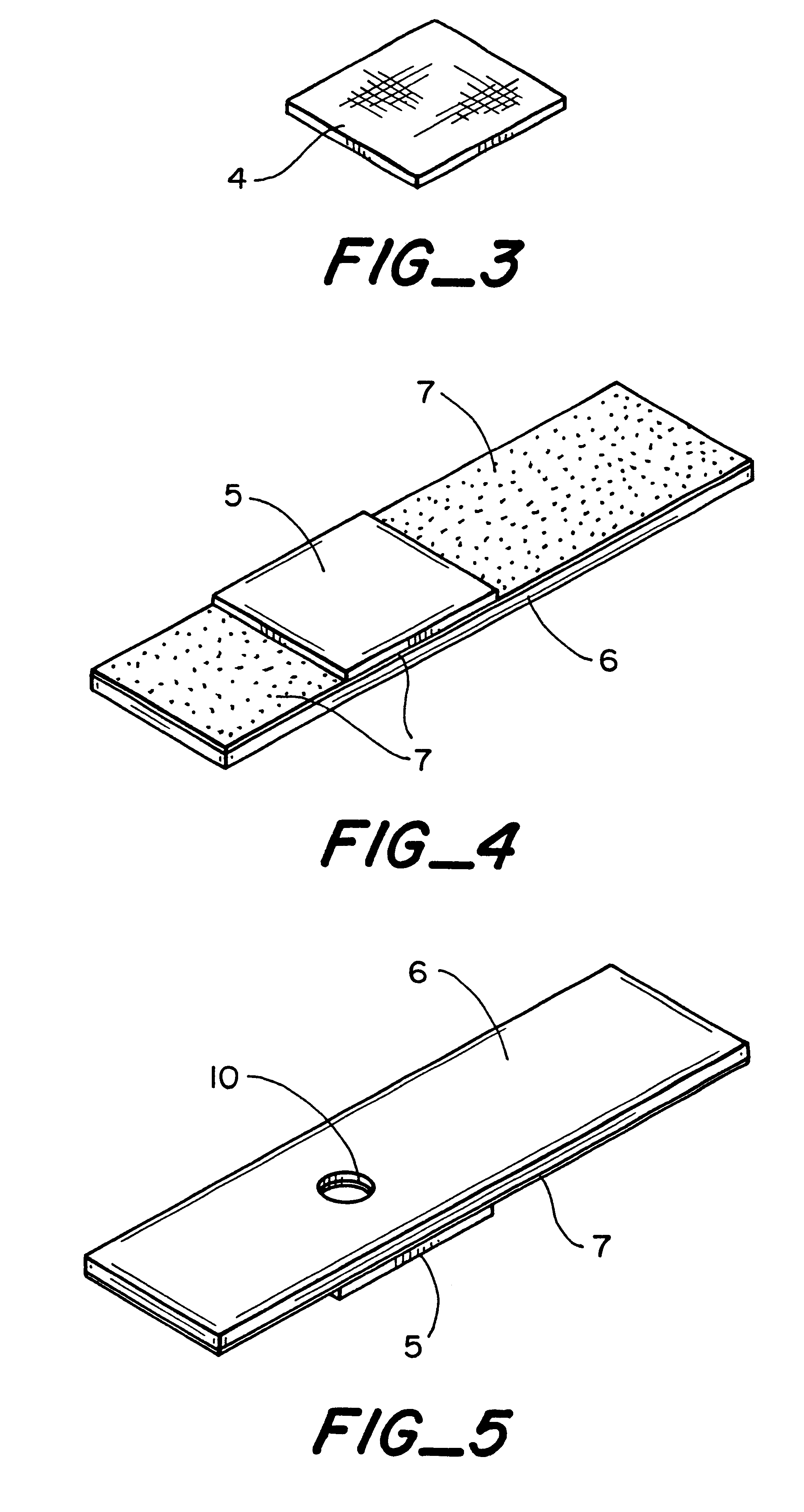
A piece of polyethersulfone membrane is uniformly coated with reagent la; the excess is squeegied off and the material is dried. The membrane is then coated with reagent 2a in the same fashion and dried. The membrane is then assembled into a test device as shown in FIG. 1. Whole blood is applied to the capillary opening and the glucose level is read from the front based on the indicator response in the test zone.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thick | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com