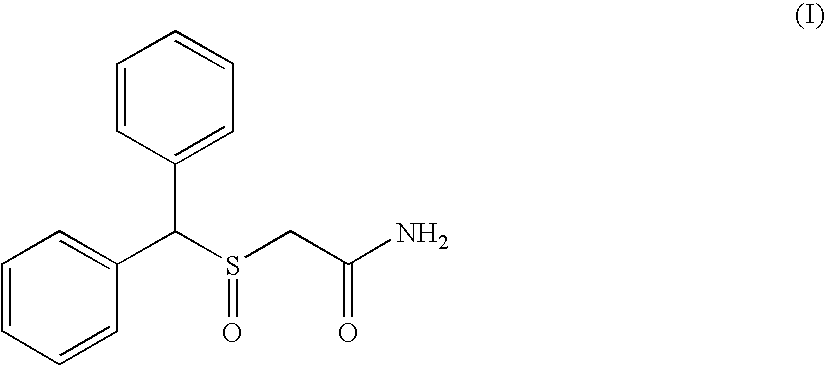
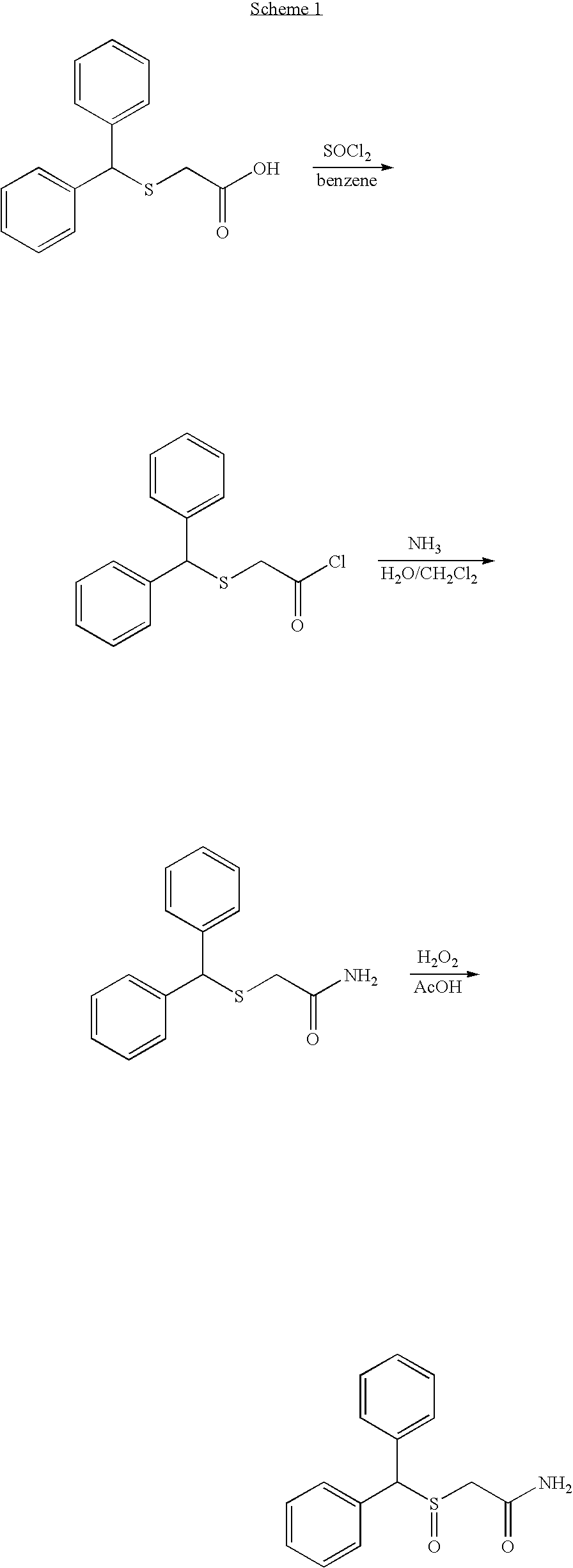
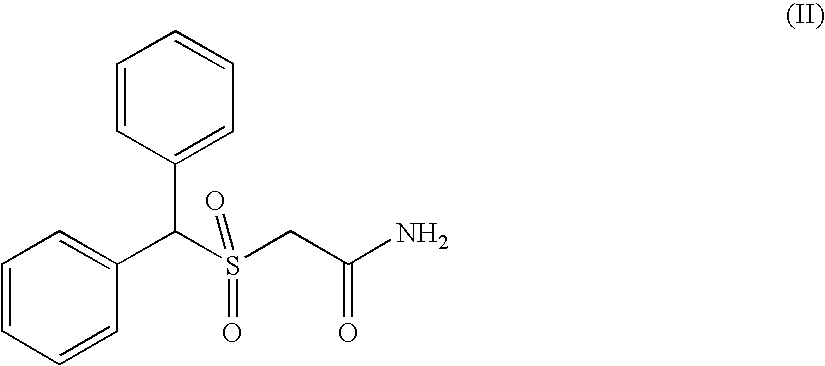
Modafinil synthesis process
a technology of modafinil and synthesis process, which is applied in the field of synthesis process of modafinil, can solve the problems of difficult control, sulphone by-product (ii) which is difficult to separate from modafinil, and the preparation process, however, has drawbacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-Liter Scale Procedure
[0107]A 1-liter reactor of type SIMULAR (Hazard Evaluation Laboratory, HEL) equipped with an impeller stirrer and a gas introduction tube was charged with 150 g of DMSAM, 450 ml of methanol and 33 mL of water. The suspension was stirred at 100 rpm and 20° C. for 10 min and then heated to 35° C. to dissolve the solids. The solution was subsequently stirred at 200 rpm for 10 min, then cooled to 25° C. and stirred at 350 rpm and at this temperature for 20 min.
[0108]46.8 g of ammonia were then introduced over 4.5 h at 25° C.
[0109]The reaction medium was left in contact for 10 h at 25° C. with stirring at 350 rpm before finally being cooled to −10° C. and then filtered over a frit of porosity 3.
[0110]The moist product was then dried under vacuum at 45° C.
[0111]Yield=89%, median=34.1 μm.
examples 2 to 5
Effect of temperature and Stirring Speed on Granulometry
example 2
Standard (Zero-Point) Experiment and Reproducibility
[0112]Conditions of standard experiment were the same as those of example 1.
[0113]The point at which the ammonia was injected, the jacket temperature, the cooling rate and the contact time at −10° C. were maintained constant during the various experiments, since these parameters had little or no influence on controlling the granulometry of the modafinil synthesized.
[0114]A standard experiment was desired in order to obtain a final granulometric median which was situated in the range 15-45 μm and thus to constitute a zero point of comparison for the subsequent experiments.
[0115]This search then culminates in the following conditions:[0116]reaction temperature T=25° C.,[0117]stirring speed SS=350 rpm,[0118]ammonia introduction time t=4.5 h.
[0119]Under these conditions the granulometric median obtained, G, was 34 μm.
[0120]This standard experiment was then repeated in order to assess its reproducibility: that was, three experiments con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com