Acylated derivative of homoharringtonine, preparation method therefor, and application thereof
a technology of acylated homoharringtonine and homoharringtonine derivative, which is applied in the field of natural medicine and pharmaceutical chemistry, can solve the problems of synthesis and application of novel mono-acylated and di-acylated homoharringtonine derivatives, and achieve the effect of improving the stability and stability of the homoharringtonine derivativ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Compound BS-HH-001
[0113]
wherein, HHT: homoharringtonine; Ac2O: acetic anhydride; DIPEA: N,N-diisopropylethylamine; DMAP: 4-dimethylaminopyridine; DCM: dichloromethane; BS-HH-001: 2,6-diacetylhomoharringtonine; BS-HH-002: 6-acetylhomoharringtonine.
[0114]Homoharringtonine HHT (125 mg, 0.23 mmol), N,N-diisopropylethylamine (444 mg, 3.44 mmol) and 4-dimethylaminopyridine (28 mg, 0.023 mmol) are dissolved in dichloromethane (2 mL). Acetic anhydride (351 mg, 3.44 mmol) is added to the mixed solution and reacted for 24 h under 35° C. The reaction solution is rinsed with water and then with saturated sodium bicarbonate, dried and concentrated. The resulted crude product is purified via Preparative Liquid Chromatography to give compound BS-HH-001 (43.3 mg, 30%) as a white solid.
[0115]LC-MS: retention time: 1.11 min (98.7%), m / z: 630.5 [M+H]+.
[0116]1H NMR (300 MHz, CDCl3): δ 6.60 (d, 2H), 5.80-5.93 (m, 3H), 5.02 (s, 1H), 3.76 (d, J=9.9 Hz, 1H), 3.67 (s, 3H), 3.60 (s, 3H), 3.03-3....
example 2
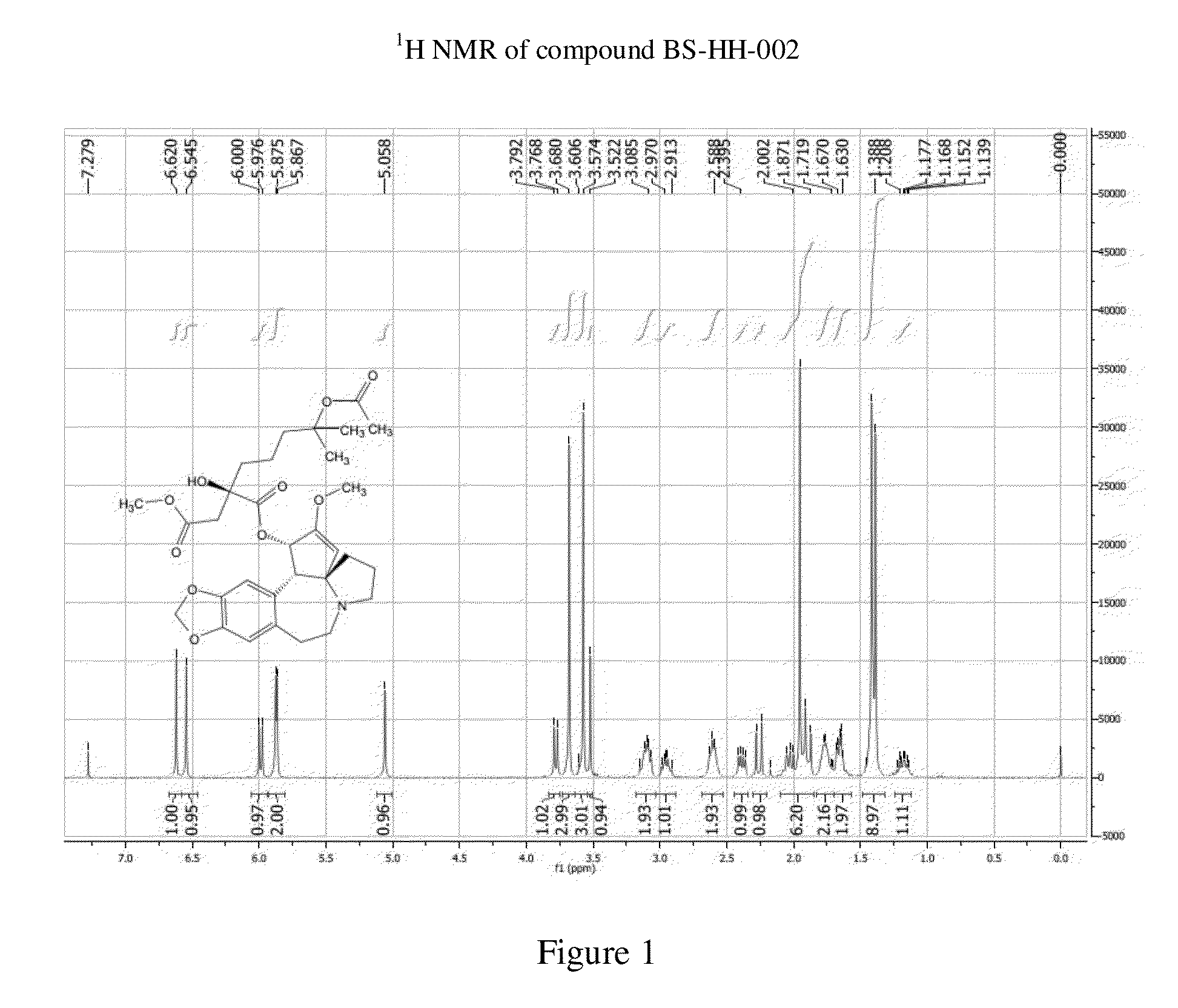
Synthesis of Compound BS-HH-002
[0117]
wherein, HHT: homoharringtonine; Ac2O: acetic anhydride.
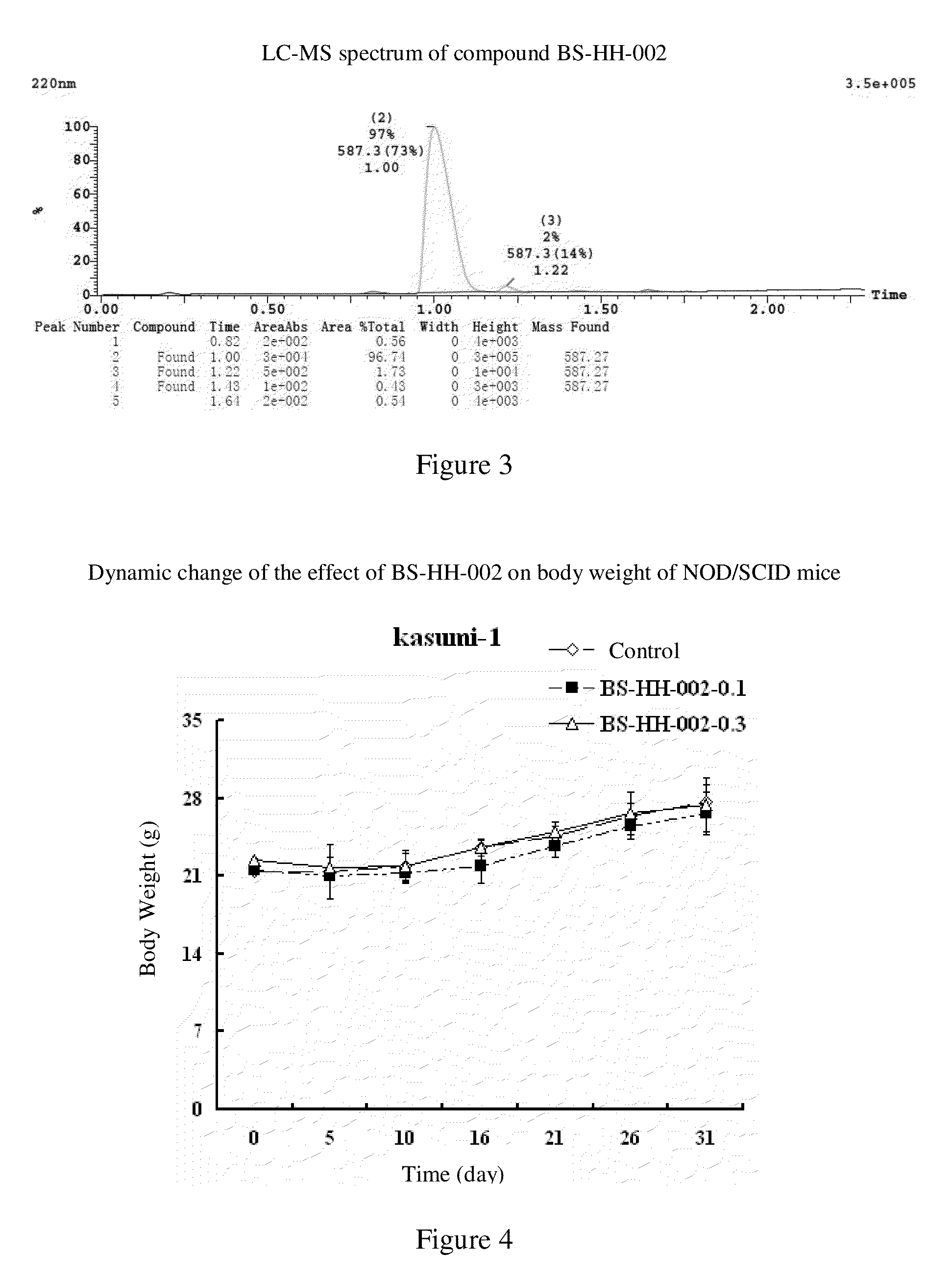
[0118]Homoharringtonine HHT (1 g, 1.84 mmol) is added to Ac2O (20 mL) and the reaction mixture is heated up to 80° C. and stirred for 13 h. After the reaction is completed, the reaction solution is concentrated to give a viscous crude product, to which diethyl ether is added for curing. Ethyl acetate is then added to the cured crude product to dissolve it. The mixture is rinsed with a saturated solution of potassium carbonate and then concentrated. The resulted crude product is purified by a Silica Gel Column Chromatography (EA:PE=1:2) to give a yellow oil, which is then recrystallized with diethyl ether to give compound BS-HH-002 (654 mg, 61%) as a white solid; the homoharringtonine HHT raw material is recovered in the meantime (354 mg).
[0119]BS-HH-002: LC-MS: retention time: 1.00 min (96.74%), m / z: 588.2 [M+H]+.
[0120]1H NMR (400 MHz, CDCl3): δ 6.62 (s, 1H), 6.55 (s, 1H), 5.99 (d, J=9.6 Hz,...
example 3
Synthesis of Compound BS-HH-077
[0122]
wherein, HHT: homoharringtonine; Yi: cyclopentylformic acid; 4-ppy: 4-(1′-tetrahydropyrrole)pyridine; DCC: dicyclohexylcarbodiimide; DCM: dichloromethane.
[0123]Homoharringtonine (110 mg, 0.2 mmol), cyclopentylformic acid (46 mg, 0.4 mmol) and 4-(1′-tetrahydropyrrole)pyridine (60 mg, 0.4 mmol) are dissolved in dichloromethane (2 mL). Dicyclohexylcarbodiimide (83 mg, 0.4 mmol) is added to the solution, which is heated and refluxed for 3 h before filtration. The filtrate is concentrated, and the resulted crude product is purified with High Performance Liquid Chromatography to give BS-HH-077 (35.9 mg, 28%) as a light yellow powdery solid.
[0124]LC-MS: retention time: 1.52 min (95.63%), m / z: 642.6 [M+H]+.
[0125]1H NMR (300 MHz, CDCl3): δ 6.63 (s, 1H), 6.59 (s, 1H), 5.99 (d, J=9.0 Hz, 1H), 5.90 (m, 2H), 5.09 (s, 1H), 3.80 (m, 1H), 3.73 (s, 3H), 3.57 (s, 3H), 3.49-3.54 (m, 1H), 2.73-3.02 (m, 2H), 2.42-2.65 (m, 3H), 2.27 (m, 1H), 2.03-2.24 (m, 3H), 1.76-1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| organic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com