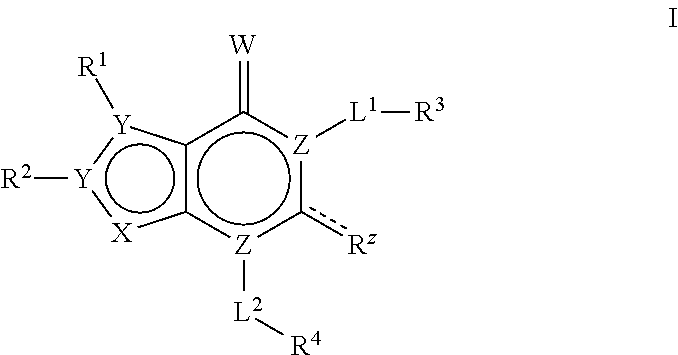
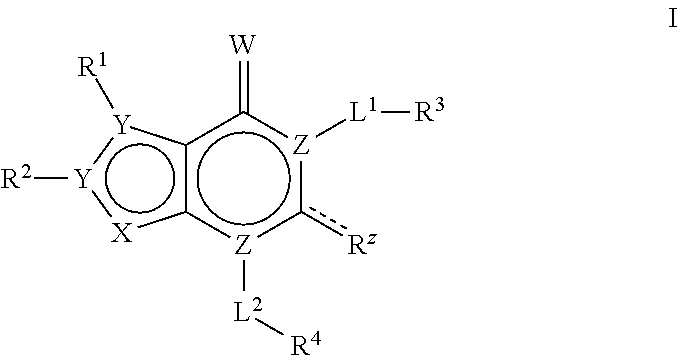
Bicyclic compounds as ACC inhibitors and uses thereof
a technology of bicyclic compounds and inhibitors, which is applied in the direction of antibacterial agents, drug compositions, and metabolic disorders, can solve the problems of increasing the risk of type-2 diabetes (t2dm), stroke and coronary heart disease, and not providing the long-term health benefit needed for weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
of Compound 2-(7-(2-methoxyphenethyl)-3-methyl-2-(oxazol-2-yl)-4-oxothieno[3,2-c]pyridin-5(4H)-yl)acetic acid, I-33
[0349]
Synthesis of Compound 1.2
[0350]Into a 250-mL 3-necked round-bottom flask under nitrogen, was placed bis(propan-2-yl)amine (15.2 g, 150.21 mmol, 1.50 equiv) in THF (100 mL). The mixture was cooled to −40° C. and butyllithium (60 mL, 1.50 equiv, 2.5 mol / L) was added slowly stirred for 30 mins. The mixture was stirred at −40° C. for 30 min. Then, the above solution was added to a solution of 2-bromo-3-methylthiophene, compound 1.1 (17.7 g, 99.96 mmol, 1.00 equiv) in THF (100 mL) at −78° C. and stirred for 30 min. N,N-dimethylformamide (100 mL) was added at −78° C. Reaction was stirred for 1 hour at −78° C. The reaction was then quenched by the addition of 1 L of NH4Cl (aq.). and extracted with 3×500 mL of ethyl acetate. Organic layers were combined and washed with 2×1000 mL of brine. Organic layer was dried over anhydrous magnesium sulfate and solvents were under vac...
example 2
of Compound 2-(7-(2-methoxyphenethyl)-3-methyl-2-(oxazol-2-yl)-4-oxothieno[3,2-c]pyridin-5(4H)-yl)acetamide, I-34
[0360]
[0361]A 25-mL 3-necked round-bottom flask kept under nitrogen, was charged with compound I-33 (40 mg, 0.08 mmol, 1.00 equiv), DCC (64 mg, 0.31 mmol, 3.73 equiv), CH2Cl2 (8 mL), NH4Cl (24.8 mg, 0.46 mmol, 5.57 equiv) and 4-dimethylaminopyridine (38 mg, 0.31 mmol, 3.74 equiv). Reaction was stirred overnight at 50° C. Upon completion, solvents were removed under reduced pressure. Crude was purified by preparative TLC and then by preparative HPLC to provide 1.9 mg (5%) of 2-(7-(2-methoxyphenethyl)-3-methyl-2-(oxazol-2-yl)-4-oxothieno[3,2-c]pyridin-5(4H)-yl)acet-amide, 1-34 as a white solid. LCMS (ES, m / z): 424 [M+H]+; 1H NMR (300 MHz, DMSO-d6): δ2.73-2.78 (t, 2H), 2.89-2.96 (m, 5H), 3.84 (s, 3H), 4.54 (s, 2H), 6.88-6.91 (t, 1H), 6.97-7.00 (dd, 2H), 7.19-7.24 (m, 3H), 7.44-7.47 (dd, 2H), 7.61 (s, 1H), 8.26 (s, 1H).
example 3
of 2-(4-(2-methoxyphenethyl)-2-(oxazol-2-yl)-5,7-dioxo-4,5-dihydrothiazolo[5,4-d]pyrimidin-6(7H)-yl)acetic acid I-35
[0362]
Synthesis of Compound 3.2
[0363]A 50-mL 3-necked round-bottom flask, was charged with compound 3.1 (200 mg, 1.16 mmol, 1.00 equiv), dichloromethane (10 mL) and triphosgene (115 mg, 0.39 mmol, 0.33 equiv). Triethylamine (352 mg, 3.48 mmol, 3.00 equiv) was the added dropwise while stirring at 0° C. The resulting solution was stirred for 1 h at 0° C. and used directly in the next step.
Synthesis of Compound 3.3
[0364]Into a 50-mL 3-necked round-bottom flask, was placed solution of compound 3.2 and tert-butyl 2-aminoacetate hydrochloride (194 mg, 1.16 mmol, 1.00 equiv). Reaction was stirred for 2 h at room temperature. Upon completion reaction was then quenched by the addition of 10 mL of NH4Cl(aq.) and extracted with 2×10 mL of dichloromethane. Organic layers were combined and concentrated under vacuum. The crude was purified using flash column chromatography to furnis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| period of time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com