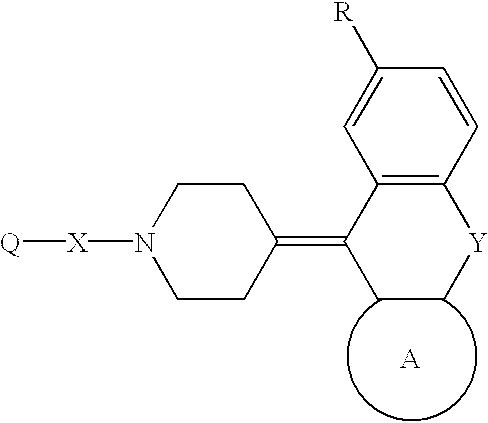
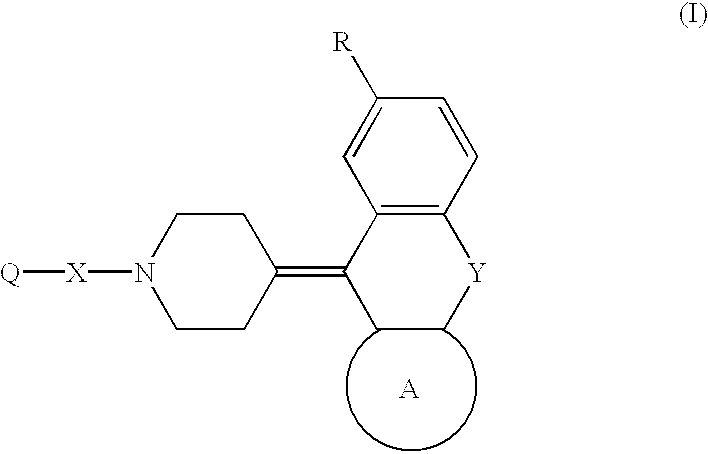
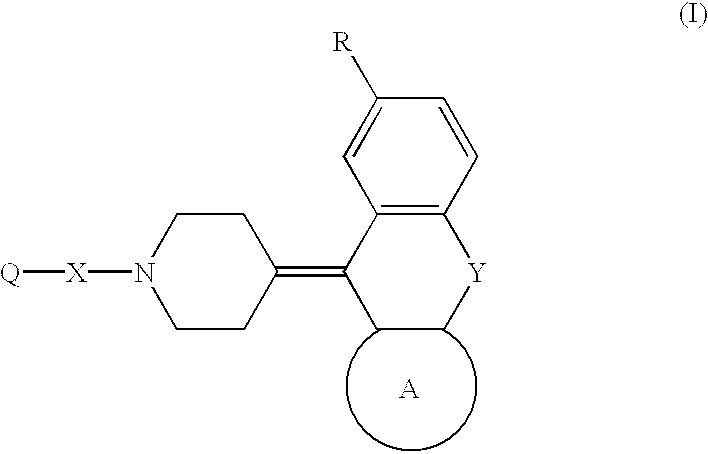
Piperidine derivatives and hypotensives containing the same
a technology of piperidine and derivatives, applied in the field of piperidine derivatives and hypotensives containing the same, can solve the problems of recent controversy over the safety or the effect of pharmaceuticals of these hypotensives, and achieve the effect of low cost and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-hexylpiperidine hydrochloride
A solution of 273 mg (1 mmol) of 4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-hexylpiperidine, 165 mg (1 mmol) of 1-bromohexane, 745 mg (5 mmols) of sodium iodide and 414 mg (3 mmols) of potassium carbonate in 20 ml of methyl isobutyl ketone was stirred and refluxed at 120.degree. C. overnight on an oil bath. After the reaction, the mixture was washed by adding 20 ml of water thereto. Then the organic phase was separated and the solvent was distilled off under reduced pressure. After purifying by silica gel column chromatography (eluent: methanol / chloroform, 1 / 100-1 / 50), the product was converted into the hydrochloride with an equimolar hydrogen chloride / dioxane solution. Amount yielded 180 mg Yield 46% TLC Rf=0.68 MS 357 (M+) NMR 0.83 (3H, t), 1.2-1.4 (6H, m), 1.7-1.9 (2H, m), 2.31 (2H, dd), 2.53 (2H, d), 2.7-2.8 (2H, m), 3.14 (2H, dd), 3.38 (2H, dd), 3.38 (2H, d), 6.92 (2H, s), 7.2-7.4 (8H, m)
Hereafter procedure...
example 2
4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-octylpiperidine hydrochloride Amount yielded 300 mg Yield 72% TLC Rf=0.71 MS 385 (M+) NMR 0.85 (3H, t), 1.2-1.4 (10H, m), 1.7-2.0 (2H, m), 2.30 (2H, dd), 2.53 (2H, d), 2.7-2.9 (2H, m), 3.13 (2H, dd), 3.38 (2H, d), 6.90 (2H, s), 7.1-7.4 (8H, m)
example 3
1-Decyl-4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)piperidine hydrochloride Amount yielded 300 mg Yield 67% TLC Rf=0.75 MS 413 (M+) NMR 0.85 (3H, t), 1.2-1.4 (14H, m), 1.7-1.9 (2H, m), 2.33 (2H, dd), 2.54 (2H, d), 2.7-2.8 (2H, m), 3.15 (2H, dd), 3.39 (2H, d), 6.92 (2H, s), 7.1-7.4 (8H, m)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com