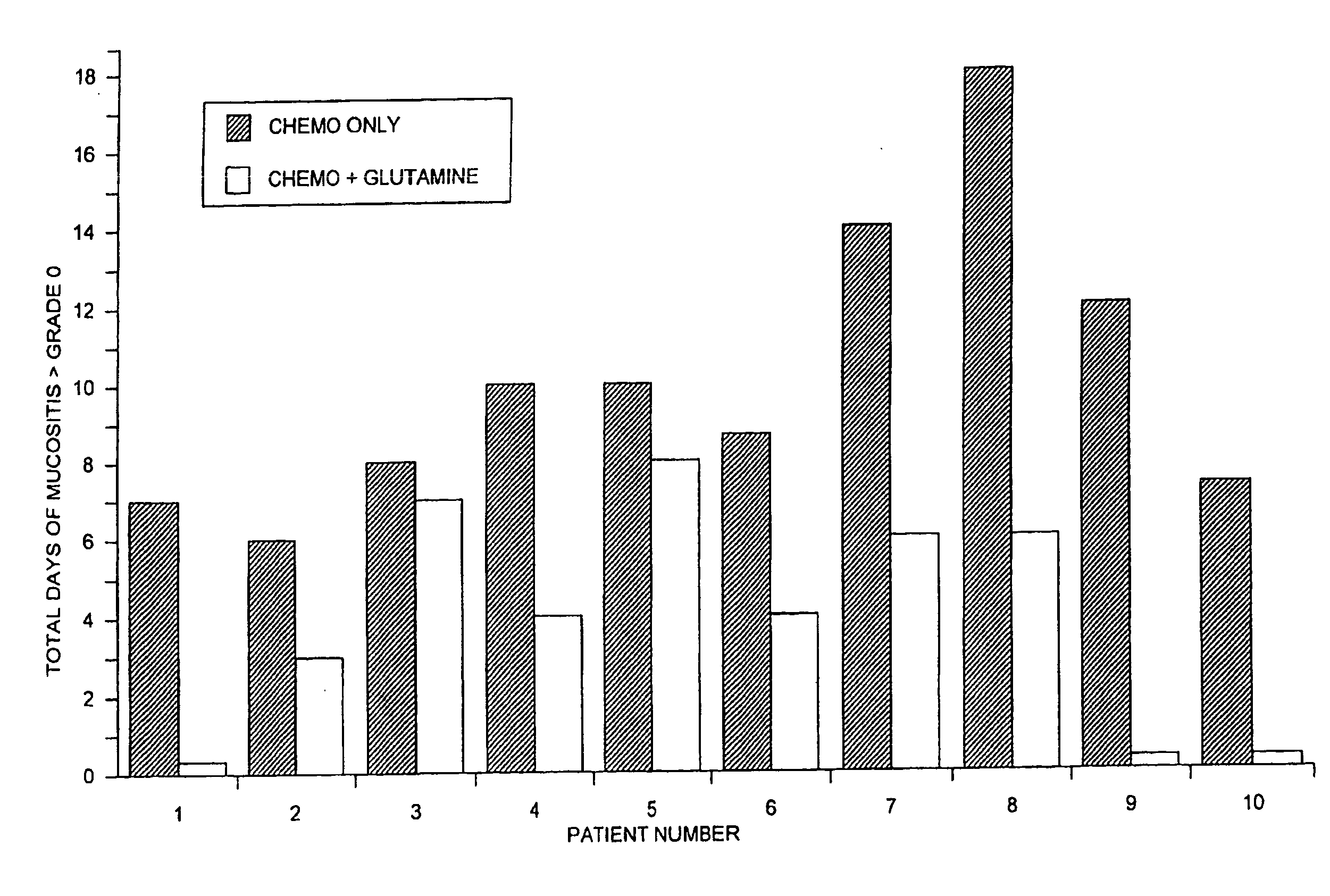
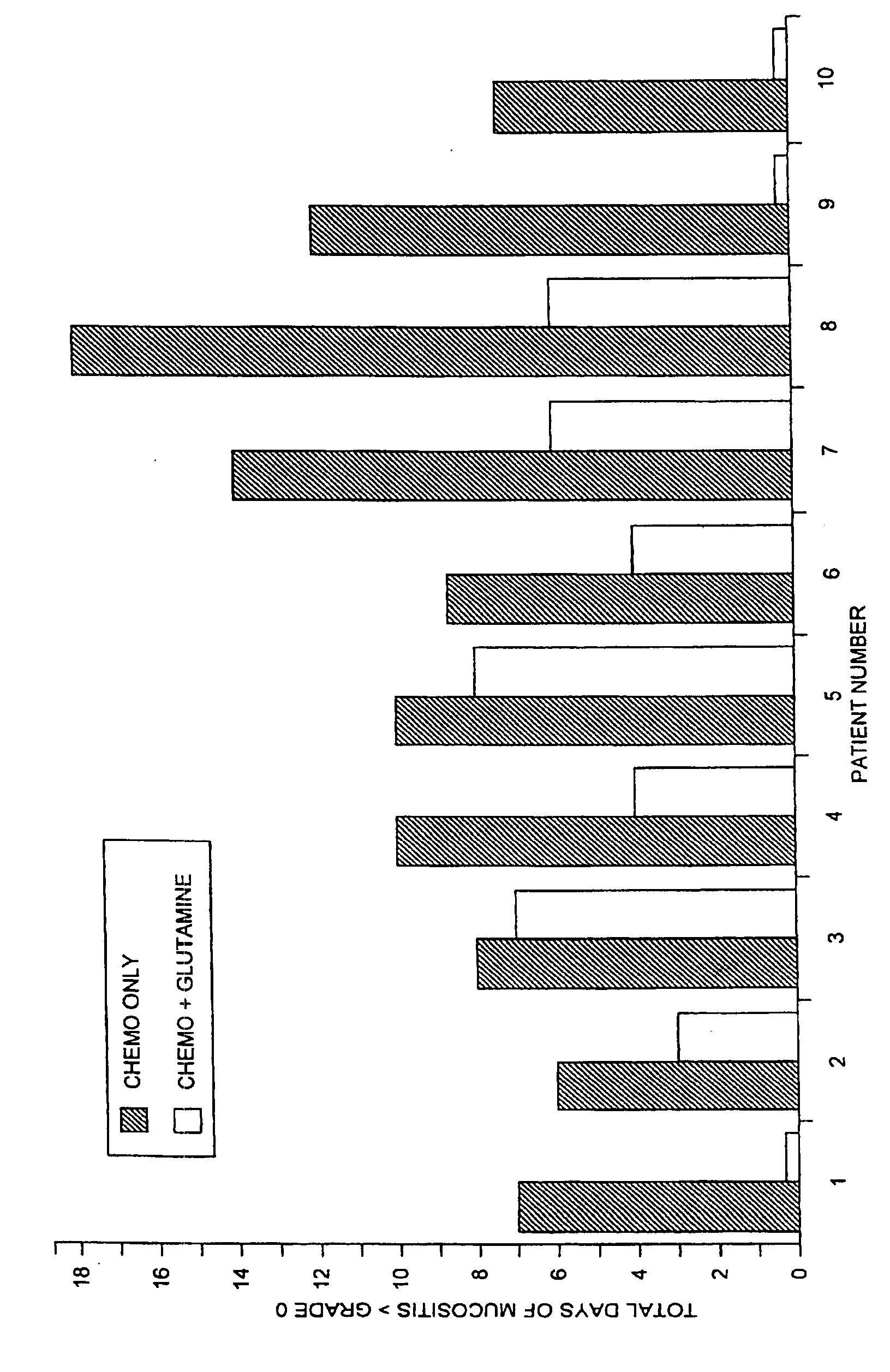
Oral Glutamine to reduce stomatitis
a glutamine and oral technology, applied in the field of oral glutamine to reduce stomatitis, can solve the problems of limiting and affecting the ability to swallow large quantities, so as to reduce the damage to the oropharyngeal mucosa from chemotherapy and minimize the damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0022]Patients experiencing mucositis of the oropharynx following a course of chemotherapy were offered the opportunity to enter this study if no other clinical parameters precluded receiving the same chemotherapy doses during the next course of treatment. Patients entering the trial received the same chemotherapy regimen as during the previous treatment, but in addition received a suspension of L-glutamine, 2 g / m2 swish and swallow twice daily, from day one of chemotherapy for 28 days or for 4 days past the resolution of any post-chemotherapy mucositis. The suspension of glutamine was prepared by mixing 50 grams of L-glutamine (supplied as a crystalline powder of Ajinomoto U.S.A., Inc., Raleigh, N.C.), with 4 parts of ORA-Sweet (Paddock Laboratories, Minneapolis, Minn.), 2 parts ORA-Plus (Paddock), and 2 parts of water to yield a suspension of 500 mg / ml L-glutamine. The final suspension contained 500 mg / ml glutamine, 30% sucrose, 2.5% glycerin, 2.8% sorbitol, 0.04% citric acid, 0.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com