Drop pill type eye drip fluid of chloramphenicol, and preparation method
A technology of chloramphenicol and eye drops, which is applied in the field of pharmaceuticals, can solve problems such as unstable quality of chloramphenicol eye drops, and achieve the effects of advanced and reliable production technology, extended validity period, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
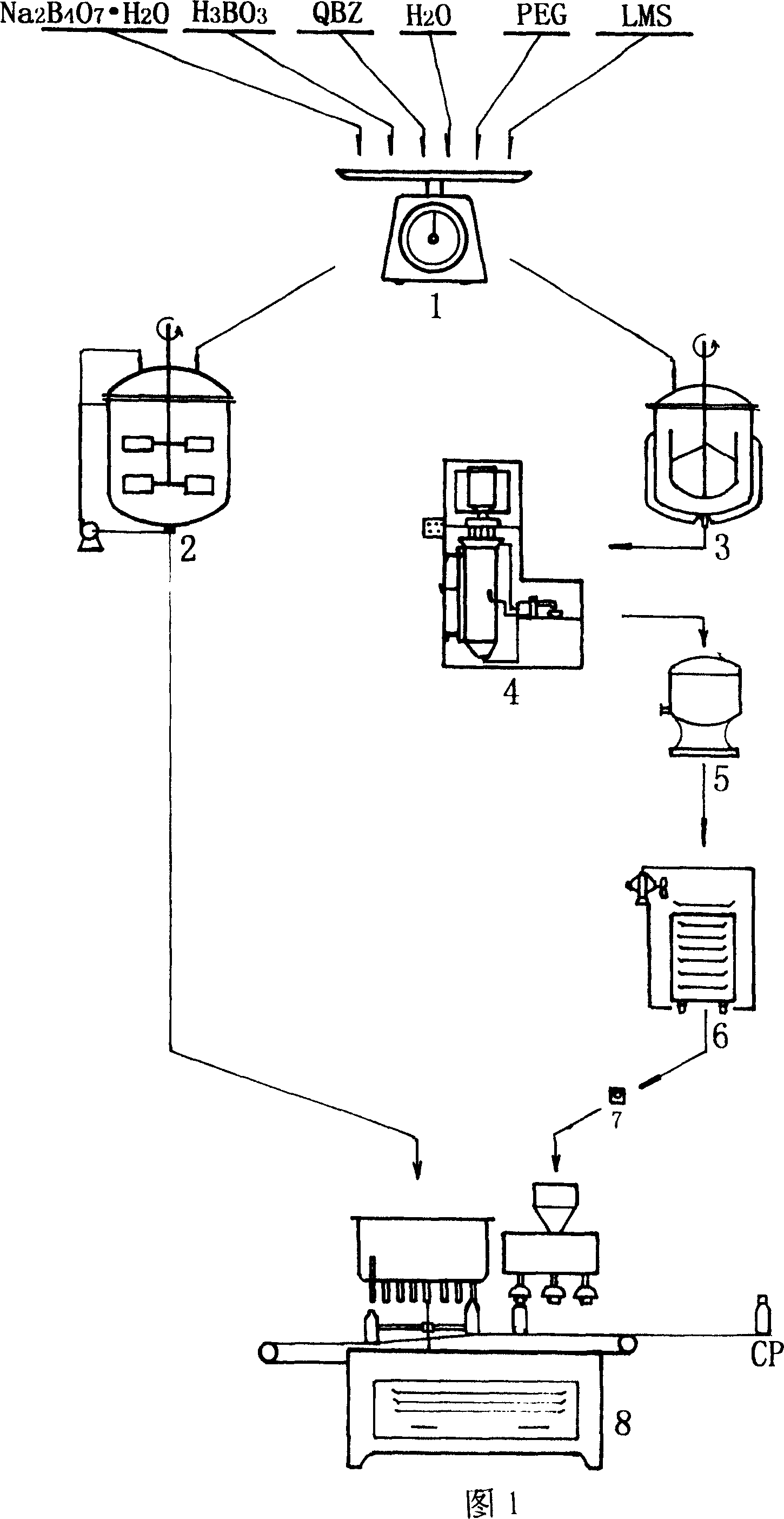
[0014] a. Weighing the ingredients, first according to the weight ratio of the prescription, chloramphenicol 12.5g, medicinal polyethylene glycol (PEG) 43.75
[0015] g, borax 1.78g, boric acid 67.05g, ethylparaben 2.49g, water for injection 5Kg, each component is weighed and prepared through weighing device 1 respectively;
[0016] b. To prepare the medicinal liquid, put the medicinal polyethylene glycol in the chemical material tank 3, heat it to 80-85°C and completely dissolve it, then add chloramphenicol, and keep the constant temperature at 80-85°C, and turn on the agitator Stir evenly to become liquid medicine;
[0017] c, make drop pills, pour the uniformly melted medicinal liquid into the drop pill machine 4, drop it into the liquid paraffin medium of 5~10° C., and quickly form it into a main pill with a weight of 56.25 mg and a content of chloramphenicol of 12.5 mg. Pills;
[0018] d, dripping pill sealing, the main medicine dripping pill that will make is first put...
Embodiment 2
[0022] a, batching weighing, first by the weight ratio of medicament prescription, chloramphenicol 20.0g, medicinal polyethylene glycol 68.8g, borax 2.88g, boric acid 107.17g, ethylparaben 4.11g, water for injection 8Kg, to Each component is weighed and prepared by weighing device 1 respectively;
[0023] b. To prepare the medicinal liquid, put the medicinal polyethylene glycol in the chemical material tank 3, heat it to 80-85°C and completely dissolve it, then add chloramphenicol, and keep the constant temperature at 80-85°C, and turn on the agitator Stir evenly to become liquid medicine;
[0024] c, make drop pills, pour the uniformly melted medicinal liquid into the drop pill machine 4, drop it into the liquid paraffin medium of 5~10° C., and quickly form it into a main pill with a weight of 88.8 mg and a chloramphenicol content of 20.0 mg. Pills;
[0025] d, dripping pill sealing, the main medicine dripping pill that will make is first put into drying machine 5 to spin d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com