Compositions and methods for mucosal delivery
A polymer, mucosal surface technology, used in drug combinations, inactive components of polymer compounds, drug delivery, etc., can solve problems such as solubility and poor bioavailability without consideration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9
[0068] Example 9 illustrates how the properties of the dosage unit vary when different hydroxymethylcellulose polymers are used. Example 10 demonstrates how the use of accelerators (exemplified by starch graft copolymers) can increase mucoadhesion up to at least 84%. In vivo studies of the dosage unit showed that it was well tolerated by patients (Example 12) and that bioavailability was improved (Example 13).
Embodiment 1-3
[0070] Embodiment 1-3: Instant Films, Composition and Related Properties
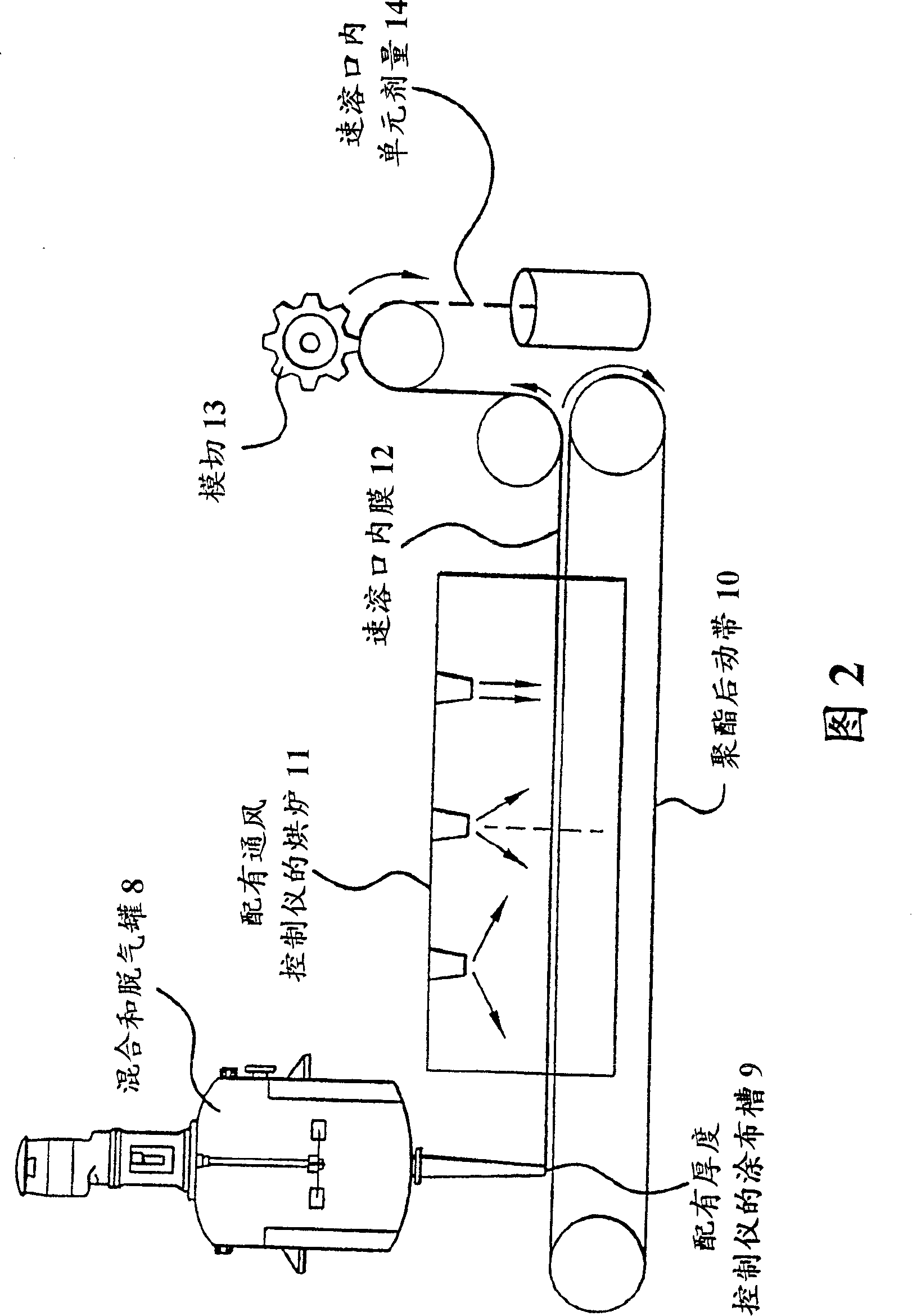
[0071] Films were prepared as follows: A homogeneous mixture of the components in the amounts given in Table 1 was prepared as a coating solution. Amounts are given in weight percent of the coating solution. The mixture was degassed in a vacuum chamber and coated on the non-siliconized side of a polyester film and dried in a hot air circulating oven to form a free-standing, non-stick flexible film. The film is then cut into dosage units for packaging.
[0072] Table 1: Formulations of instant films using several different hydrocolloids
[0073] Composition: coating solution%
Example 1
Example 2
Example 3
Pullalan (P-20)
%(weight)
17.5
Methocel E5% (by weight)
21.06
POLYOX WSR N-10% (by weight)
1.8
PVA(Vinol 125)%(weight)
1.5
Cellulose gum % (weight)
8.1
Propylene glycol % (weigh...
Embodiment 4-8
[0081] Embodiment 4-8: Hydroxypropyl methylcellulose-based fast-dissolving oral lining containing therapeutic agent
[0082] Membranes were prepared according to Examples 1-3. The therapeutic agent is added to the homogeneous mixture (coating solution) prior to film formation.
[0083] table 5
[0084] Composition (coating solution)
Example 4
Example 5
Example 6
Example 7
Example 8
1.4
2.92
3.71
Estradiol
1.49
Mint
1.0
1.0
1.0
1.0
1.0
Methocel E5 (HPMC)
21.06
21.06
21.06
21.06
21.06
1.0
1.0
1.01
1.0
1.0
0.8
0.8
0.8
0.8
0.8
0.7
0.7
0.7
0.7
0.7
40
1.0
1.0
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com