Method for preparing Lithium transition metal composite oxides from carbonate propodosoma
A lithium transition metal and composite oxide technology, which is applied in the preparation of alkali metal oxides, carbonates/acid carbonates, alkali metal compounds, etc., can solve the problems of difficult practical operations, reduced lithium content, and difficult operations. Difficulty and other problems, to achieve the effect of narrow particle size distribution, long service life and large specific capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention is described in detail below in conjunction with accompanying drawing:
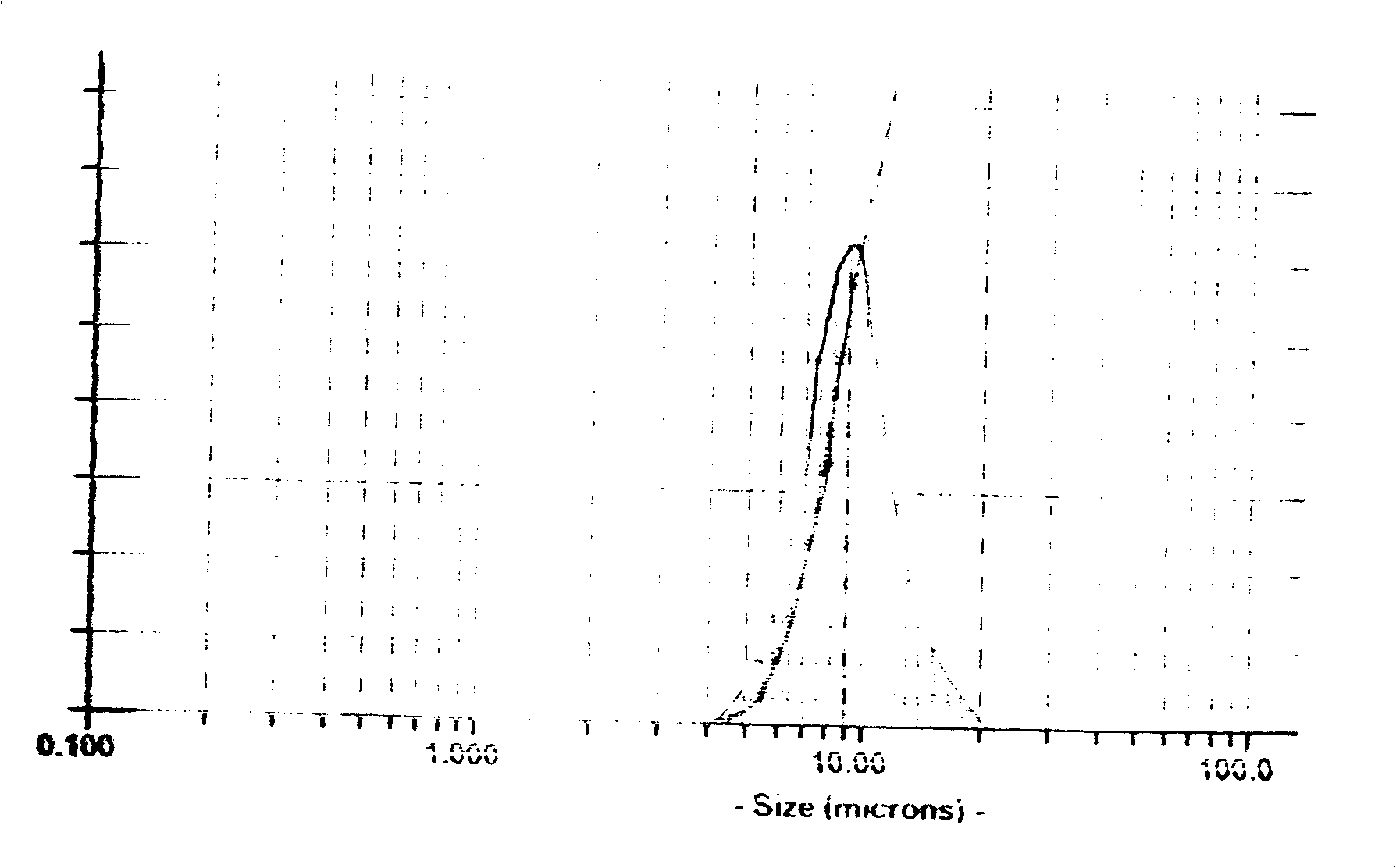
[0032] The synthetic chemical formula is Ni 0.4 co 0.2 mn 0.4 CO 3 As an example, measure and prepare 1.0mol / l cobalt, nickel, manganese mixed sulfate solution, 1.0mol / l Na 2 CO 3 Aqueous solution, 1.0mol / l NaOH aqueous solution, add the above solution into the reactor at the same time, keep the reaction speed at 45°C, control the pH value at 8.5-9, stir the reaction time at 40 rpm for 10 hours; filter, wash, dry dry treatment to get Ni 0.4 co 0.2 mn 0.4 CO 3 Prebody, with figure 1 It can be seen from the electron microscope photos that the precursor body is non-spherical, uniform in size, dispersed without aggregates, figure 2 It shows a narrow particle size distribution with an average particle size of 10um.
[0033] The synthetic chemical formula is LiNi 0.4 co 0.2 mn 0.4 o 3 For example, add Ni 1-x-y CO X mn y CO 3 Prebody, Li 2 CO 3 Mix the dry mater...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com