Ketoconazole eye drops and fabricating method thereof
A manufacturing method and technology of eye drops, which are applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of long stirring time, increased manufacturing costs, complicated operation process, etc., and achieve the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
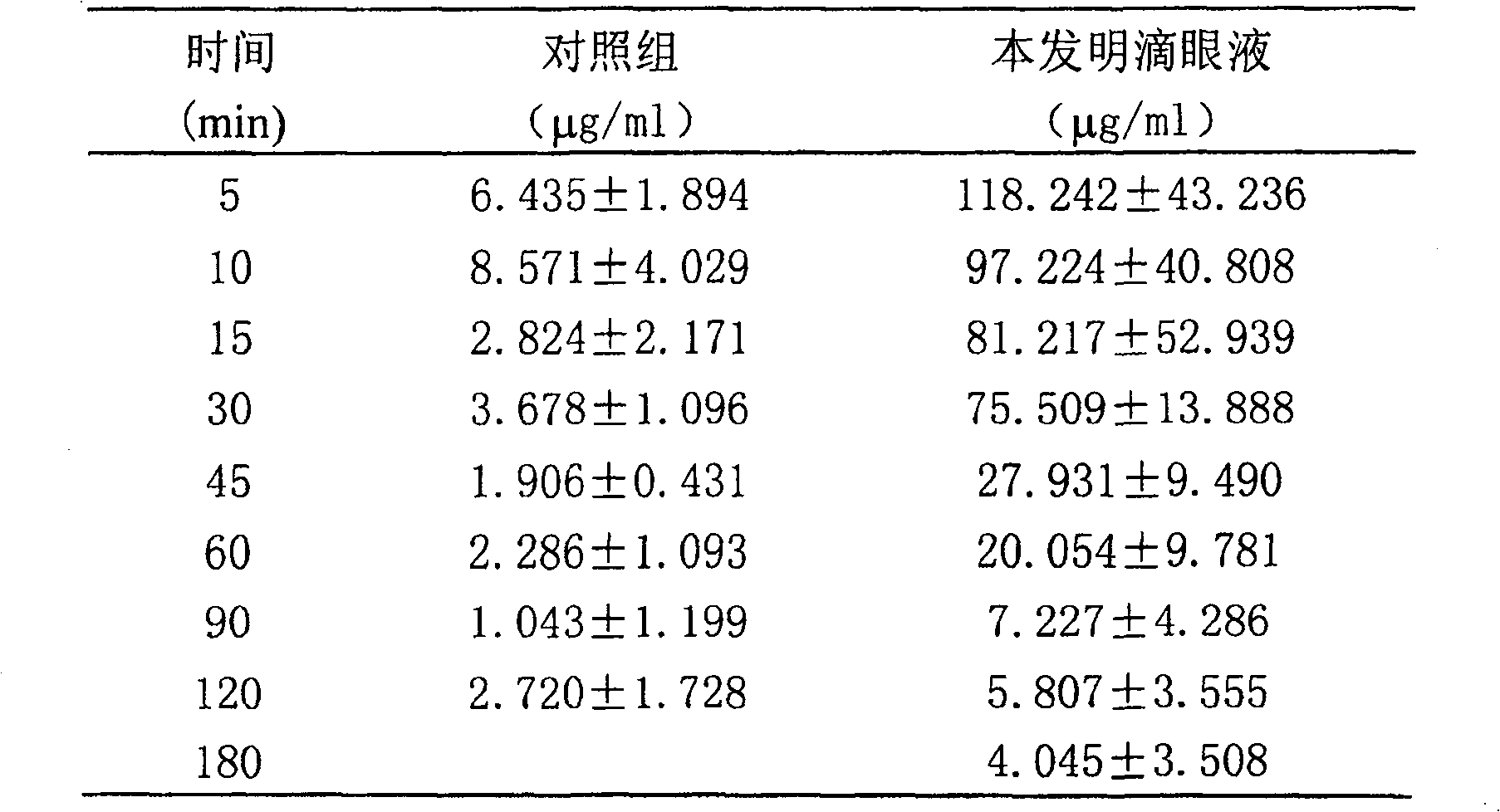
[0019] Example 1: Weigh 30 grams of hydroxypropyl-β-cyclodextrin, add 100 milliliters of water for injection to it, stir at room temperature to dissolve it, and obtain a transparent solution A; weigh 5 grams of ketoconazole powder, and then take A Solution 15 ml, ketoconazole powder was added to the solution, and then 10 ml of 1mol / L hydrochloric acid was added thereto, stirred and dissolved at room temperature to obtain a transparent solution B; while stirring, solution B was added to solution A, and gradually added 10 ml of 1 mol / L sodium hydroxide, and adjust the pH value to 5.5-8 to obtain solution C; under stirring, add 0.1 g of bacteriostatic agent benzalkonium chloride and 10 g of polyvinyl alcohol to solution C, and continue Stir and heat the solution to reach 80°C until the polyvinyl alcohol dissolves, add water for injection to make up to 1000ml, then filter, sterilize with circulating steam at 100°C, and distribute it in 5ml or 8ml eye drops bottles under aseptic con...
Embodiment 2
[0022] Example 2: Weigh 500 grams of hydroxypropyl-β-cyclodextrin, add 400 milliliters of water for injection into it, and stir to dissolve it at a temperature of 30° C. to obtain a transparent solution A; weigh 50 grams of ketoconazole powder , and then take 45 ml of solution A, add ketoconazole powder to the solution, then add 100 ml of 1mol / L hydrochloric acid to it, stir and dissolve at 30°C to obtain a transparent solution B; add solution B while stirring into solution A, and gradually add 90 ml of 1mol / L sodium hydroxide to adjust the pH value to 5.5-8 to obtain solution C; under stirring, add bacteriostatic agent ethylparaben 0.3 g and 10 grams of polyvinyl alcohol, continue to stir and heat to make the solution temperature reach 50°C until the polyvinyl alcohol dissolves, add water for injection to make up to 1000 ml, then filter, sterilize with circulating steam at 100°C, and divide under aseptic conditions Packed in 5ml or 8ml eye drops bottle.
Embodiment 3
[0023] Example 3: Weigh 300 grams of hydroxypropyl-β-cyclodextrin, add 300 milliliters of water for injection into it, stir and dissolve it at a temperature of 10°C to obtain a transparent solution A; weigh 40 grams of ketoconazole powder , and then take 25 ml of solution A, add ketoconazole powder to the solution, then add 50 ml of 1mol / L hydrochloric acid to it, stir and dissolve at a temperature of 10°C to obtain a transparent solution B; add solution B while stirring into solution A, and gradually add 60 ml of 1mol / L sodium hydroxide to adjust the pH value to 5.5-8 to obtain solution C; under stirring, add bacteriostatic agent ethylparaben 0.4 g and 15 grams of polyvinyl alcohol, continue to stir and heat to make the solution temperature reach 40°C until the polyvinyl alcohol dissolves, add water for injection to make up to 1000 ml, then filter, sterilize with circulating steam at 100°C, and divide under aseptic conditions Packed in 5ml or 8ml eye drops bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com