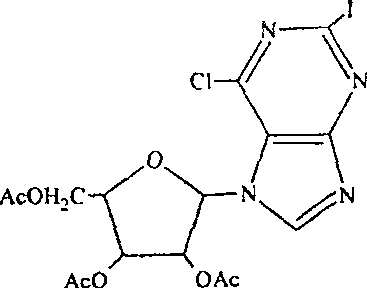
Purification of 2-iodo-6-chloro-9-beta(2',3',5'-trioxyacetyl-D-furanose)purine
A technology of trioxyacetyl and ribofuranose, applied in sugar derivatives, organic chemistry, etc., can solve the problems of long cycle, difficult operation of silica gel column chromatography, high cost, etc., and achieve short cycle, stable product quality and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
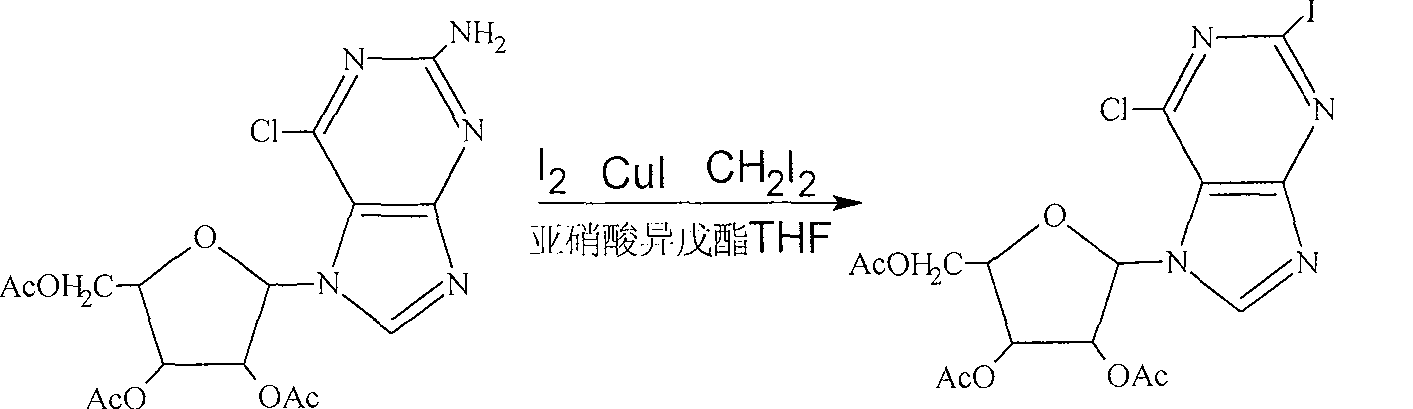
[0021] 24mmol (10.24 g), iodine 12mmol (6.08g), cuprous iodide 12.6mmol (4.8g), diiodomethane 20ml, tetrahydrofuran 120ml, stirred at room temperature, then added 10ml of isoamyl nitrite to reflux, reacted for 45 minutes, and kept at 70°C , add 3 g of activated carbon (fineness 200-400 mesh), stir for 15 minutes, cool to room temperature, filter, add 500 ml of ethyl acetate to dissolve the filtrate after evaporating the solvent, wash with 100 ml of 10% sodium thiosulfate solution, and separate the organic phase , dried with anhydrous magnesium sulfate for 30 minutes, and the filtered filtrate was evaporated to dryness to obtain 14.2 g of crude product.
[0022] Add 14.2 g of the crude product to 60 ml of ethyl acetate, heat to 60°C, and after the crude product is completely dissolved, add 60 ml of n-hexane (the volume ratio of solvent ethyl acetate to n-hexane is 1:1), recrystallize, filter, and dry. Obtain 2-iodo-6-chloro-9-β-(2', 3', 5'-trioxyacetyl-D-ribofuranose) purine p...
Embodiment 2
[0024] With heating, stirring, add 2-amino-6-chloro-9-β-(2', 3', 5'-trioxyacetyl-D-ribofuranose) purine 30.6g ( 72mmol), iodine 18.3g (36mol), cuprous iodide 14.4g (37.8mmol), diiodomethane 60ml, tetrahydrofuran 360ml, stirred at room temperature, then added 30ml of isoamyl nitrite to reflux, reacted for 45 minutes, and kept at 70°C , add 6 g of activated carbon (fineness 200-400 mesh), stir for 45 minutes, cool to room temperature, filter, add ethyl acetate 1000 ml to dissolve after the filtrate evaporates the solvent, wash with 250 ml of 15% sodium thiosulfate solution, and separate the organic phase , dried with anhydrous magnesium sulfate for 30 minutes, and the filtered filtrate was evaporated to dryness to obtain 40.1 g of crude product.
[0025] Add 40.1 g of the crude product to 150 ml of ethyl acetate, heat to 60°C, and after the crude product is completely dissolved, add 225 ml of n-hexane (the volume ratio of solvent ethyl acetate to n-hexane is 1:1.5), recrystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com