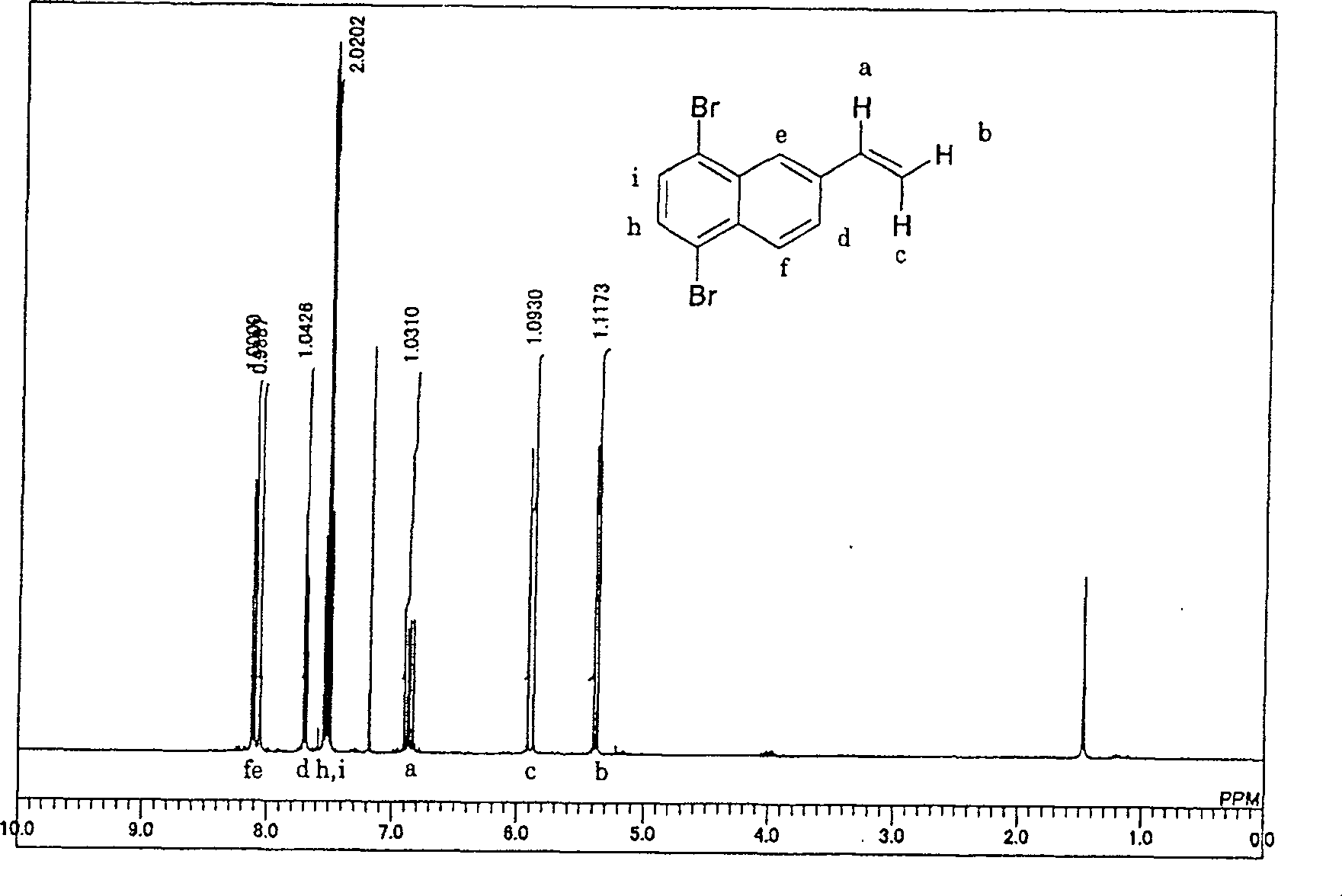
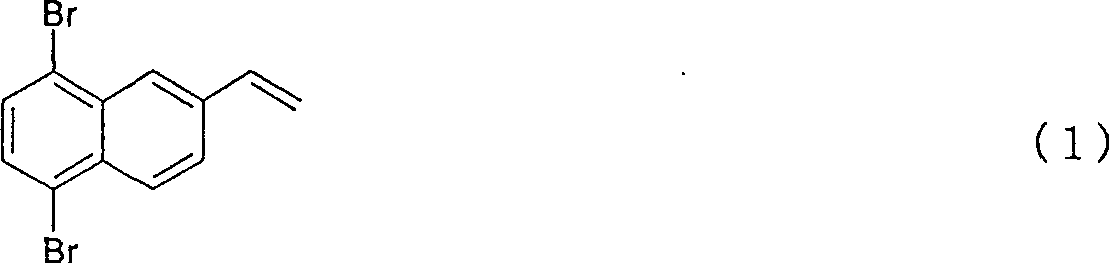5,8-dibro-2-vinylnaphthaline and preparation method thereof
A technology of vinyl naphthalene and vinyl, applied in 5 fields, can solve the problems of no teaching, impossible modification of aromatic ring, etc., and achieve the effect of low dielectric constant, low dielectric loss and low water absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 50 g (purity: 93%) of 2-vinylnaphthalene was added to 400 g of methylcyclohexane, dissolved, and kept at -10°C. Subsequently, bromine was slowly added dropwise under the condition that the reaction temperature did not exceed -5°C to carry out bromination of the vinyl group. The reaction liquid started to develop color from the moment when bromine, which was substantially equimolar to 2-vinylnaphthalene, was dropped, and it was confirmed that the bromine addition reaction of the vinyl group was terminated. After confirming the coloring, once the dripping of bromine was stopped, the temperature was raised to 20° C., and then 1 g of reduced iron was added. After the temperature was raised, the dropwise addition of bromine was started again, and a total of 4 mol times of bromine was added to 2-vinylnaphthalene in 3 hours, and further, heating was continued for 30 minutes to complete bromination.
[0025] After the obtained reaction solution was cooled to room temperature, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com