Quality control method of bone sinew medicinal pill preparation
A detection method, the technology of Gujin Wan, which is applied in the field of quality control of medicines and Gujin Wan preparations, can solve problems such as backward quality control standards, difficult product quality control, and inability to effectively control the quality of Gujin Wan preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
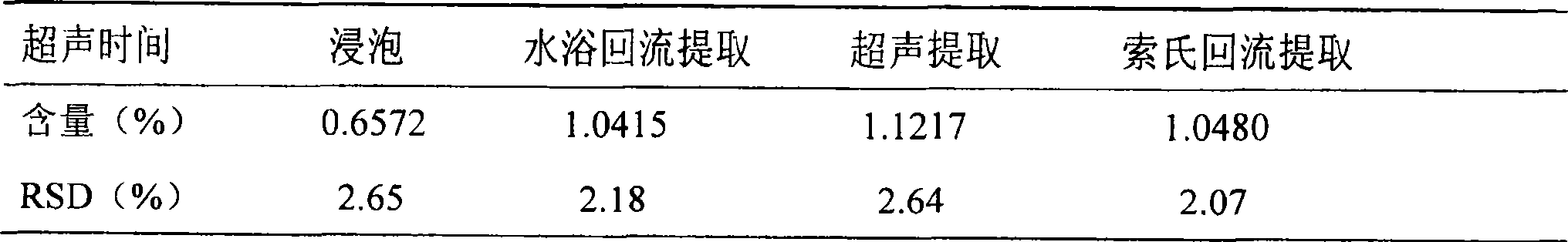
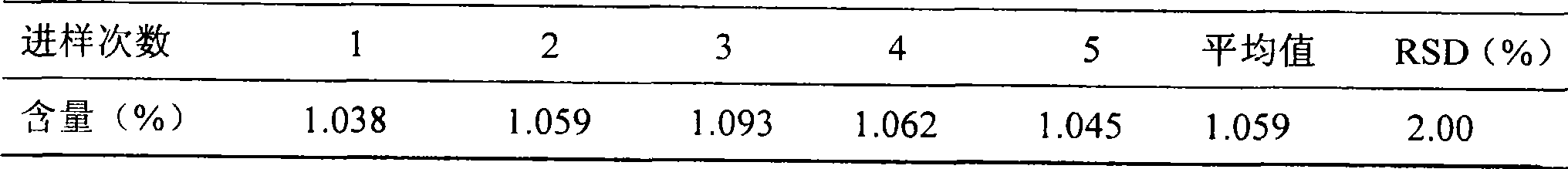
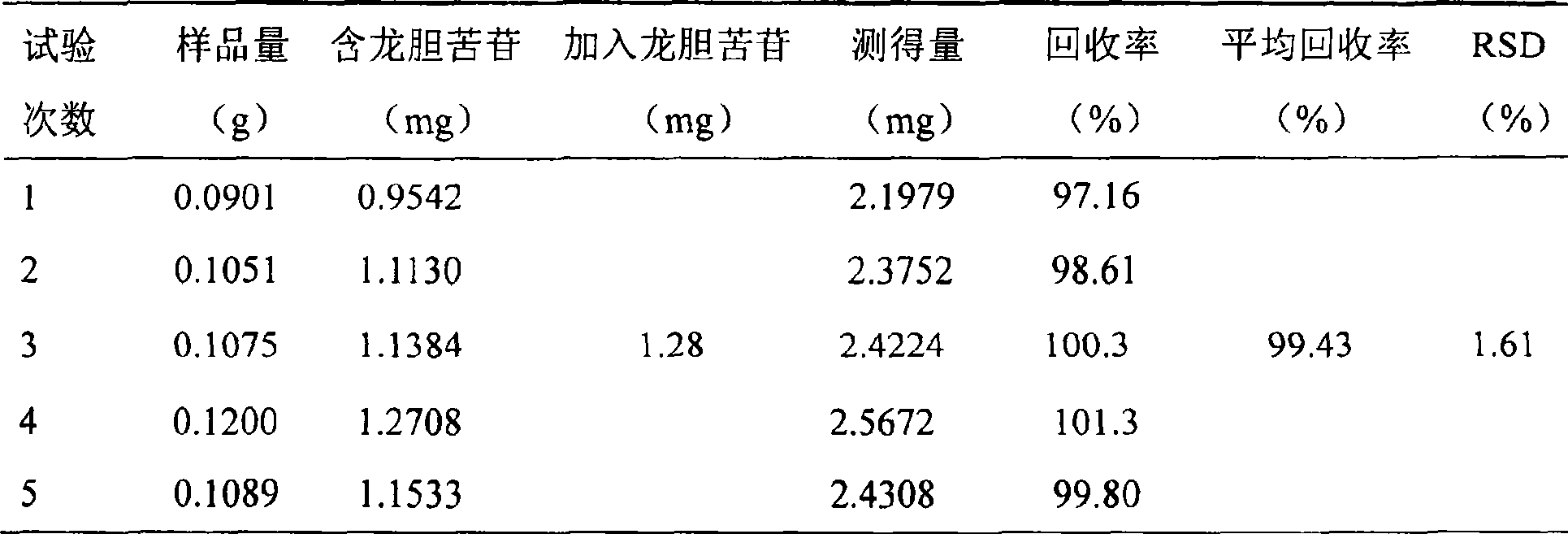
[0111] Quality control methods for Gujinwan capsules, tablets or granules.
[0112] To observe the traits, the steps are;
[0113] For capsules: the contents are brown; smell, bitter and astringent;
[0114] For tablets; the drug is brown; smell, bitter, astringent;
[0115] For granules: the product is brown granules;
[0116] To identify the contents, the steps are:
[0117] (1) Take capsules, tablets or granules respectively and observe under a microscope: gelatinized starch granules are light yellow or nearly colorless; hypodermal sclerenchyma cells are green and yellow, and the cells are polygonal, square or long. , the wall is slightly curved, lignified, some become beaded and thickened, and the pits are fine; the stone cells are light yellow, sub-round or oblong, with a diameter of about 60 μm, the wall is thicker, and the pits are fine; the diameter of the threaded vessel is 16 ~32μm; many starch granules, single round, semicircular or round polygonal, 4~30μm in di...
Embodiment 2
[0127] To observe the traits, the steps are:
[0128] For capsules: the contents are brown; smell, bitter and astringent;
[0129] For tablets: the drug is brown; the smell is bitter and astringent;
[0130] For granules: the product is brown granules;
[0131] To identify the contents, the steps are:
[0132] (1) Take capsules, tablets or granules respectively and observe under a microscope: gelatinized starch granules are light yellow or nearly colorless; hypodermal sclerenchyma cells are green and yellow, and the cells are polygonal, square or long. , the wall is slightly curved, lignified, some become beaded and thickened, and the pits are fine; the stone cells are light yellow, sub-round or oblong, with a diameter of about 60 μm, the wall is thicker, and the pits are fine; the diameter of the threaded vessel is 16 ~32μm; many starch granules, single round, semicircular or round polygonal, 4~30μm in diameter; compound grains are composed of more than 2~10 sub-grains; re...
Embodiment 3
[0143] To observe the traits, the steps are:
[0144] For capsules: the contents are brown; smell, bitter and astringent;
[0145] For tablets: the drug is brown; the smell is bitter and astringent;
[0146] For granules: the product is brown granules;
[0147] To identify the contents, the steps are:
[0148] (1) Take capsules, tablets or granules respectively and observe under a microscope: gelatinized starch granules are light yellow or nearly colorless; hypodermal sclerenchyma cells are green and yellow, and the cells are polygonal, square or long. , the wall is slightly curved, lignified, some become beaded and thickened, and the pits are fine; the stone cells are light yellow, sub-round or oblong, with a diameter of about 60 μm, the wall is thicker, and the pits are fine; the diameter of the threaded vessel is 16 ~32μm; many starch granules, single round, semicircular or round polygonal, 4~30μm in diameter; compound grains are composed of more than 2~10 sub-grains; re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com