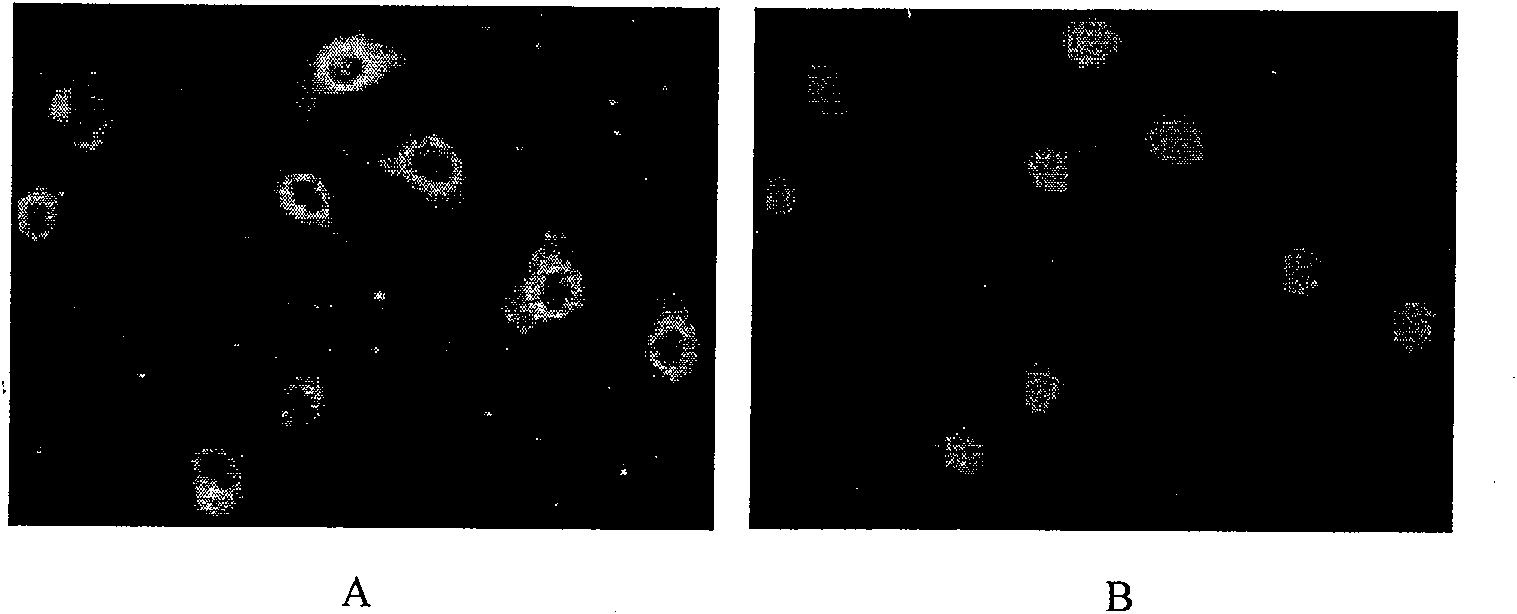
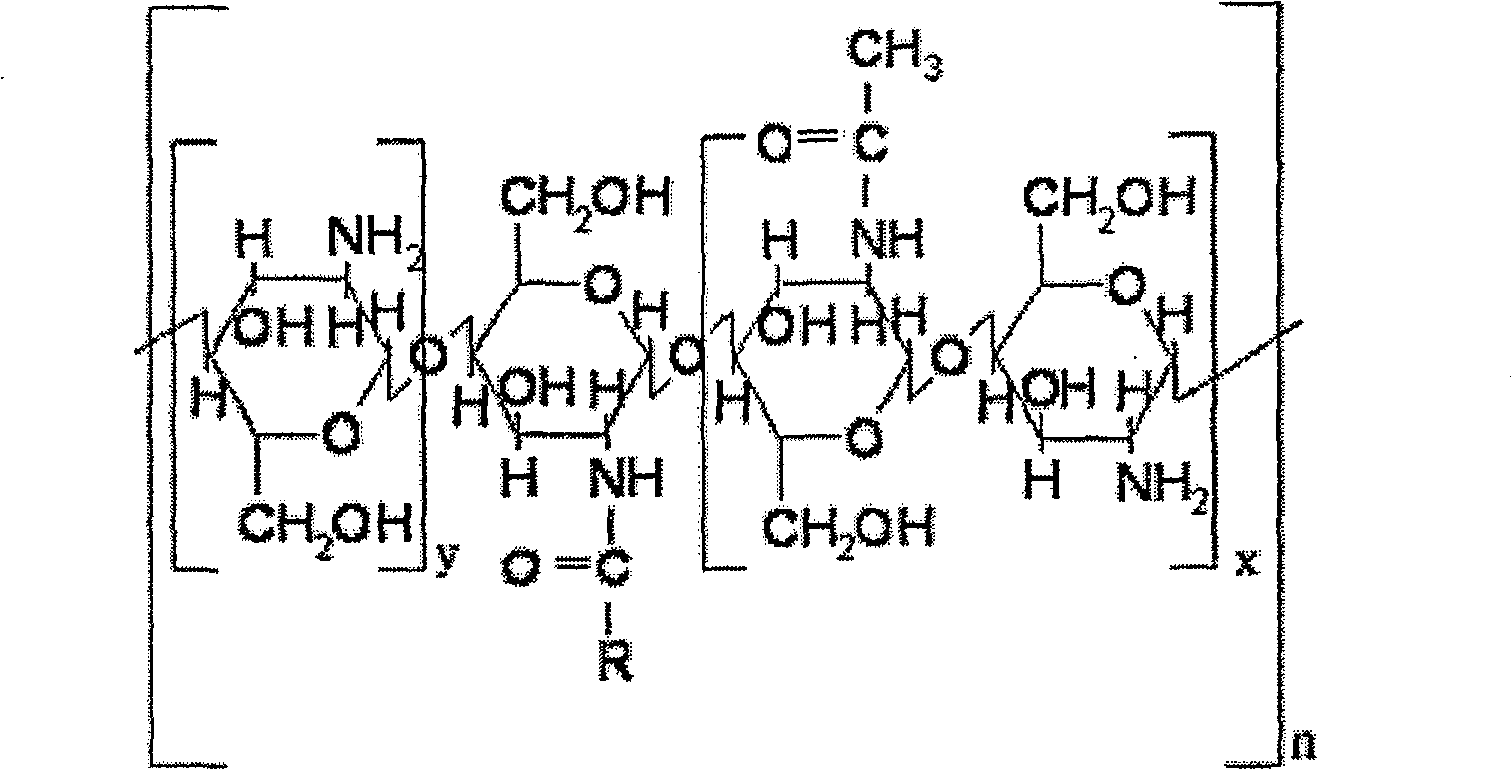
Synthesis method for subcellular organelle target directional Chitosan oligosaccharide-aliphatic acid grafting matter
A synthetic method and chitosan oligosaccharide technology, applied in the preparation of multi-hydrophobic inner chitosan oligosaccharides-fatty acid graft micelles, the application field of nano-scale micellar particle carriers in the field of life sciences, can solve the limitation of hydrophobic modification Problems such as the application of chitosan micelles, high viscosity, and the difficulty of preparing chitosan microparticles and nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] 1. Preparation of low molecular weight chitosan (chitooligosaccharide)
[0024] Take commercially available chitosan (70% degree of deacetylation) with a molecular weight of 550kDa, stir for 2 hours at 55°C and pH5. : 100 (w / w) cellulase was added to degrade chitosan. The degree of degradation of chitosan was controlled by viscosity method. The resulting chitosan degradation solution is filtered to remove impurities, and ultrafiltration is used for fractionation using ultrafiltration membranes with molecular weights of 10 kDa and 30 kDa. The ultrafiltrate with a molecular weight between 10kDa and 30kDa was freeze-dried to obtain chitosan oligosaccharide with a deacetylation degree of 70% and a molecular weight of 22.4kDa.
[0025] 2. Preparation of chitooligosaccharide-fatty acid graft and determination of physical and chemical properties
[0026] Take the above 0.0001mol chitosan oligosaccharide, dissolve it in 100ml distilled water, prepare the aqueous solution of ...
example 2
[0039] 1. Preparation of low molecular weight chitosan (chitooligosaccharide)
[0040] Get commercially available chitosan (90% degree of deacetylation) with a molecular weight of 450kDa, stir for 2 hours at 55°C and pH5. : 100 (w / w) cellulase was added to degrade chitosan. The degree of degradation of chitosan was controlled by viscosity method. The obtained chitosan degradation solution is filtered to remove impurities, and the ultrafiltration membranes with molecular weights of 3kDa, 10kDa, 30kDa and 50kDa are used for ultrafiltration classification. The fractionated ultrafiltrate was freeze-dried to obtain chitosan oligosaccharides with a degree of deacetylation of 90% and molecular weights of 1.0 kDa, 22.2 kDa, 33.4 kDa, 54.6 kDa and 101.2 kDa.
[0041] 2. Preparation of oligochitosan-stearic acid graft and determination of physical and chemical properties
[0042] Dissolve the above 0.0001mol oligochitosaccharides in 100ml distilled water to prepare an aqueous solutio...
example 3
[0052] 1. Preparation of low molecular weight chitosan (chitooligosaccharide)
[0053] Get commercially available chitosan (100% degree of deacetylation) with a molecular weight of 750kDa, stir for 2 hours at 55°C and pH5. : 100 (w / w) cellulase was added to degrade chitosan. The degree of degradation of chitosan was controlled by viscosity method. The resulting chitosan degradation solution is filtered to remove impurities, and ultrafiltration is used for fractionation using ultrafiltration membranes with molecular weights of 10 kDa and 30 kDa. The fractionated ultrafiltrate was freeze-dried to obtain chitooligosaccharides with a deacetylation degree of 100% and a molecular weight of 25.8 kDa.
[0054] 2. Preparation of oligochitosan-stearic acid graft and determination of physical and chemical properties
[0055] Take the above 0.0001mol chitosan oligosaccharide, dissolve it in 100ml distilled water, prepare the aqueous solution of chitosan oligosaccharide, add 0.01mol N-h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com