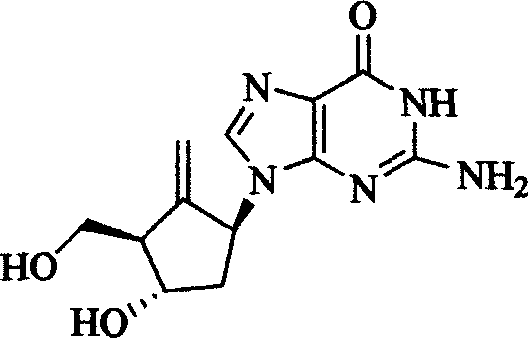
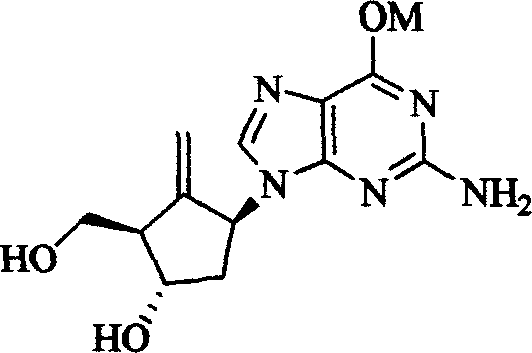
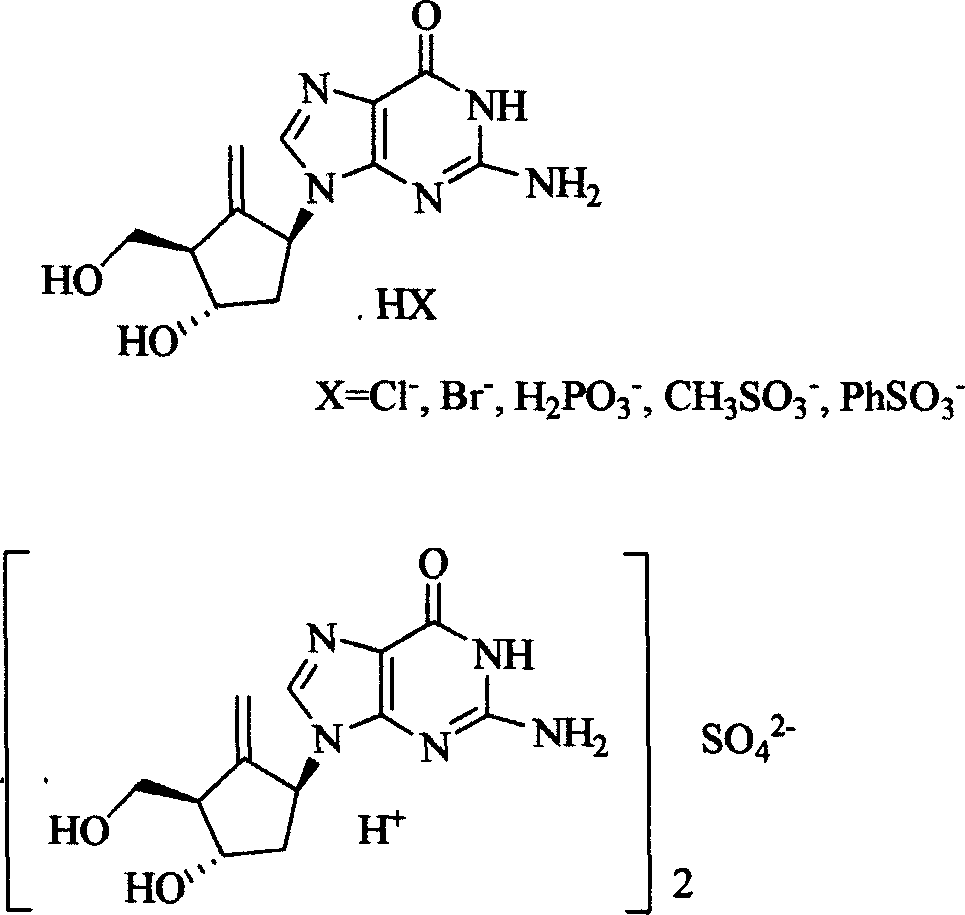
Entecavir salt, and preparation
A technology of entecavir and cavir base salt, which is applied in the field of pharmaceutical preparations and anti-hepatitis B virus, and can solve the problems that the entecavir base salt is not stable enough and does not teach the preparation method of entecavir acid addition salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of entecavir hydrochloride salt
[0047] 3g entecavir (prepared by the method of document US5,206244 example K) is dissolved in 20 milliliters of dimethylacetamide (DMA), slowly add 10 milliliters of 2N hydrochloric acid ethanol solution, constantly stir the reaction solution, react at room temperature for 1 After 1 hour, the reaction product was filtered, and the solid product was washed with a little anhydrous ether. The product was vacuum-dried (40° C., 30 mmHg) and recrystallized from anhydrous ethanol solvent to obtain 2.4 g (yield 80%) of entecavir hydrochloride salt white crystals (melting point greater than 250° C.). Elemental analysis result C 12 h 16 N 5 o 3 Cl, calcd: C, 45.94; H, 5.14; Cl, 11.30; N, 22.32; O, 15.30. Experimental values: C, 45.90, H5.11, Cl, 11.35, N, 22.30.
[0048] The NMR spectrum data are as follows: 1 H NMR (DMSO-d 6 , 300MHz, ppm), 11.89(brs, 1H), 7.46(s, 2H), 8.86(s, 1H), 5.22(t, J=12Hz, 2H), 5.1...
Embodiment 2
[0050] Embodiment 2: the preparation of entecavir bromate salt
[0051] 2.0g entecavir (prepared according to the method of file US5,206244 example K) was dissolved in 20 milliliters of dimethylacetamide (DMA), slowly added 10 milliliters of 2N hydrobromic acid ethanol solution, constantly stirring the reaction solution, at room temperature After reacting for 1 hour, the reaction product was filtered, and the solid product was washed with a little anhydrous ether. The product was vacuum-dried (40° C., 30 mmHg) and recrystallized from anhydrous ethanol solvent to obtain 1.7 g (yield 85%) of entecavir bromate salt as white crystals (melting point greater than 260° C.). Elemental analysis result C 12 h 16 N 5 o 3 Br, calcd: C, 40.24; H, 4.50; Br, 22.31; N, 19.55; O, 13.40. Found: C, 40.30, H, 4.52, Br, 22.35, N, 19.50. The nuclear magnetic resonance spectrum data is identical with embodiment 1.
Embodiment 3
[0052] Embodiment 3: the preparation of entecavir sulfate salt
[0053] 2.0g entecavir (prepared according to the method of document US5,206244 example K) was dissolved in 20 ml of dimethylacetamide (DMA), slowly added 10 ml of 1N ethanol sulfuric acid solution, the reaction solution was constantly stirred, and reacted at room temperature After 1 hour, the reaction product was filtered, and the solid product was washed with a little anhydrous ether. The product was vacuum-dried (40° C., 30 mmHg) and recrystallized from absolute ethanol to obtain 1.5 g (yield 75%) of entecavir sulfate white crystals (melting point greater than 280° C.). Elemental analysis results [C 12 h 16 N 5 o 3 ] 2 SO 4 , calcd: C, 44.17, H, 4.94; N, 21.46; O, 24.52; S, 4.91. Experimental values: C, 44.13; H, 4.91; N, 21.41; S, 4.87. The nuclear magnetic resonance spectrum data is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com