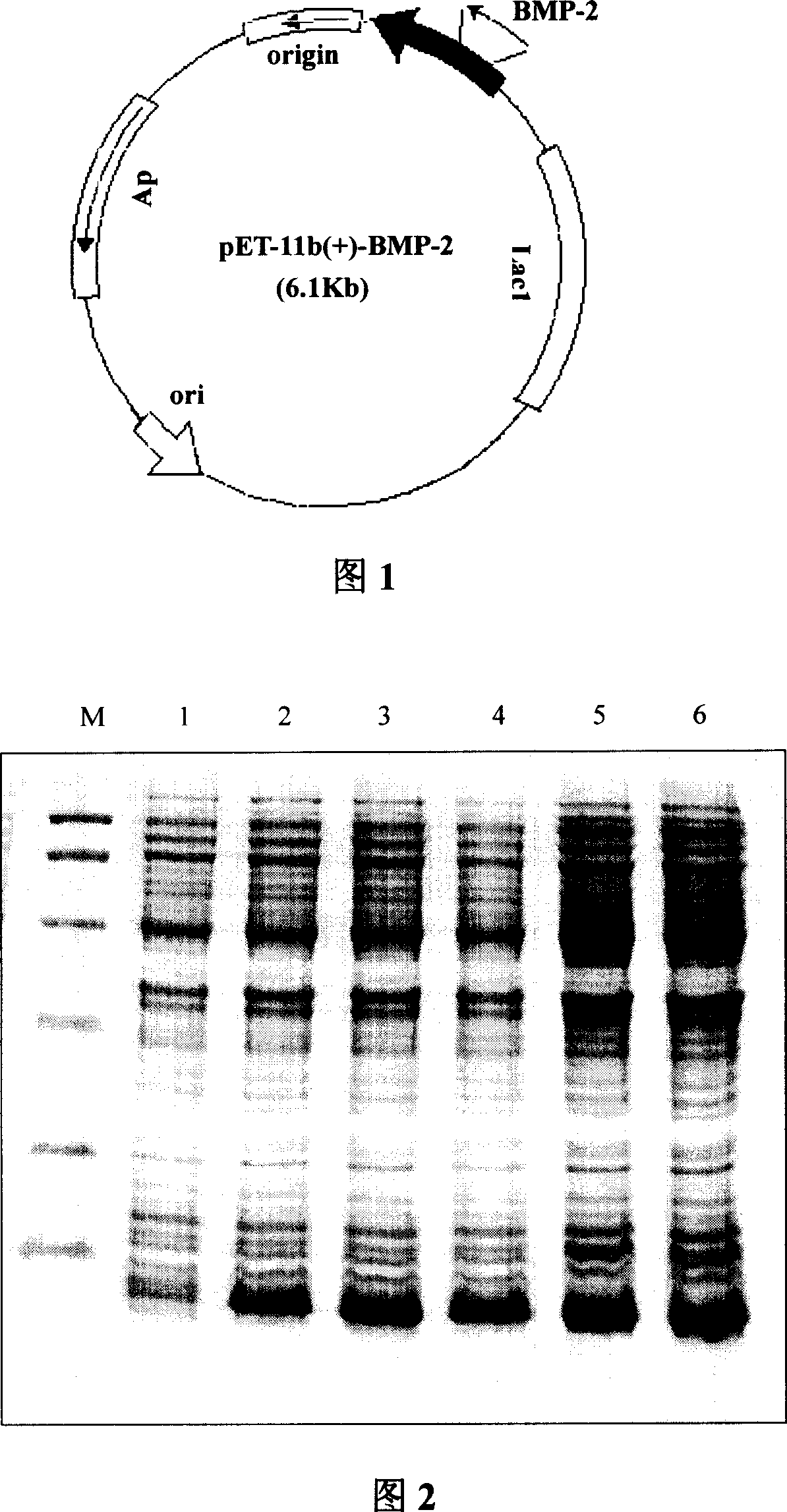
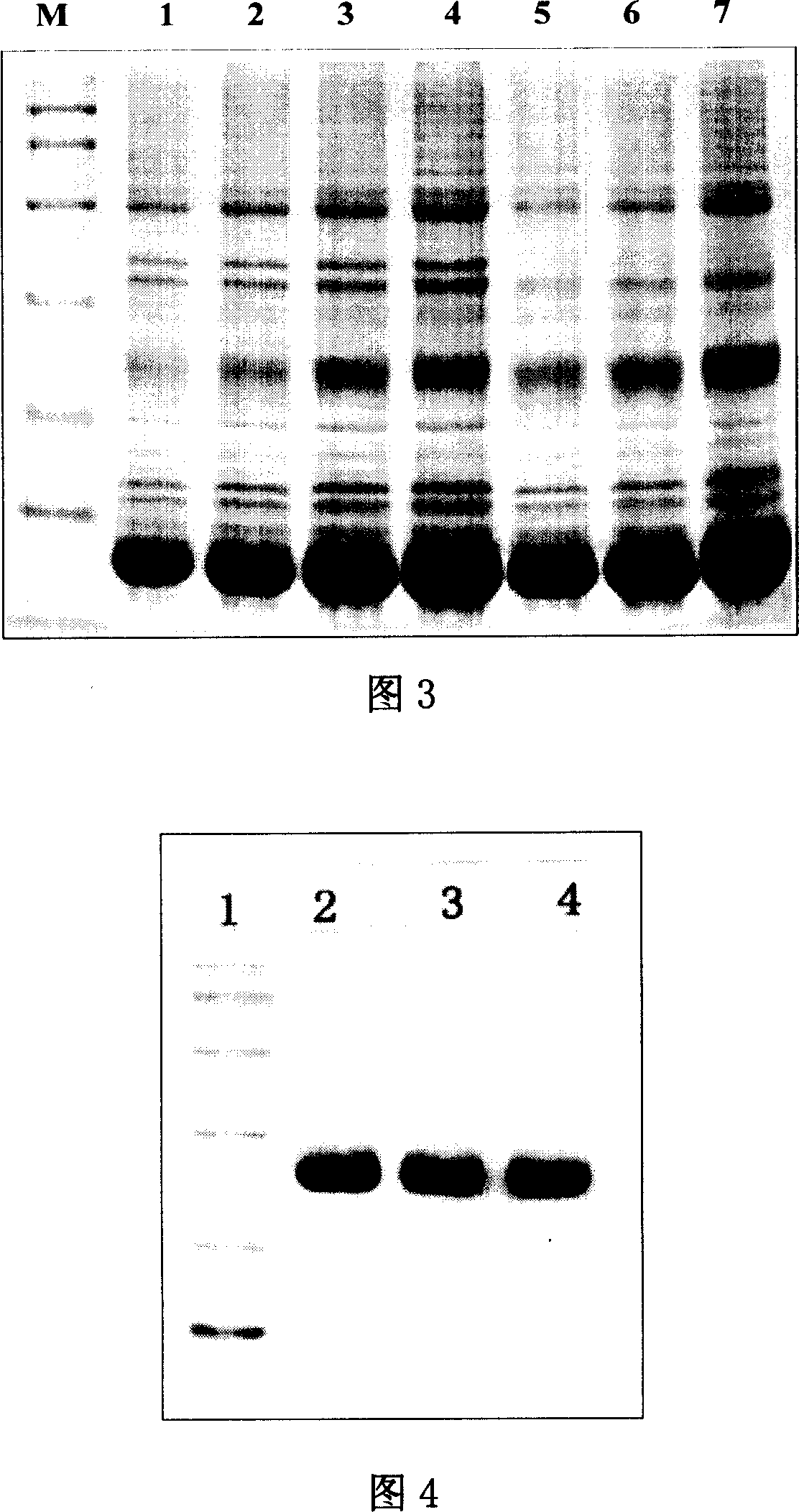
Method for preparing maturation peptide of morphogenesis protein - 2 of human bones
A morphogenetic protein and mature peptide technology, which is applied in the preparation methods of peptides, biochemical equipment and methods, and botanical equipment and methods, etc., can solve the problems of long human bone morphogenetic protein-2 being unable to realize industrialized production, and achieve the cost The effect of low, high expression and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] 1. Acquisition of BMP-2 gene
[0048] Take 0.5 g of fetal liver tissue, grind it with a homogenizer, and extract total RNA with Trizol reagent. PCR primers were designed according to the BMP-2 gene sequence published by Genebank:
[0049] Primer 1: 5'TATA CATATG (NdeI restriction site) CAA GCC AAA CAC AAA CAGCGG.
[0050] Primer 2: 5'TGCA GAATTC (EcoR I restriction site) CGT CGACTC TAGAGTATA.
[0051] When designing the sequence, before the start codon ATG, an Nde I restriction site was added, and at the 3' end of the gene was a non-coding region sequence, so that the gene 3' end stop codon and the regulatory sequence stopped translation during expression.
[0052] Reverse transcription to obtain BMP-2 cDNA: In a 0.5ml micro-reaction tube, add 200pmol / L 3' end primer and 15μg total RNA to 24μl reaction solution, heat at 70°C for 5 minutes, add first-strand buffer, RNsin, AMV reverse Enzymes, dNTPs, and the mixed reaction were reacted at 42°C for 1 hour.
[0053] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com