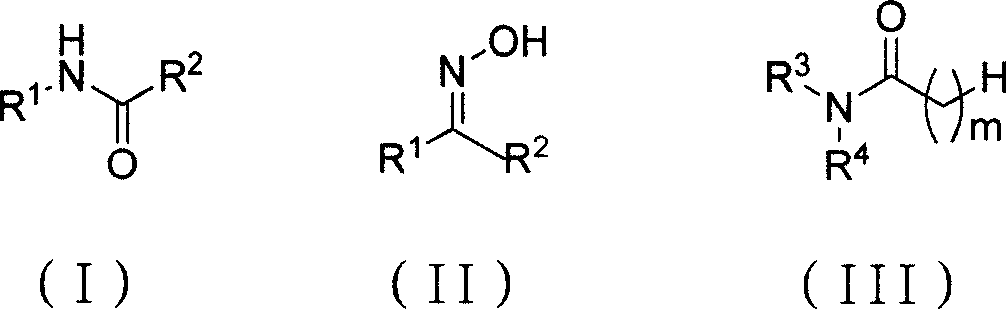
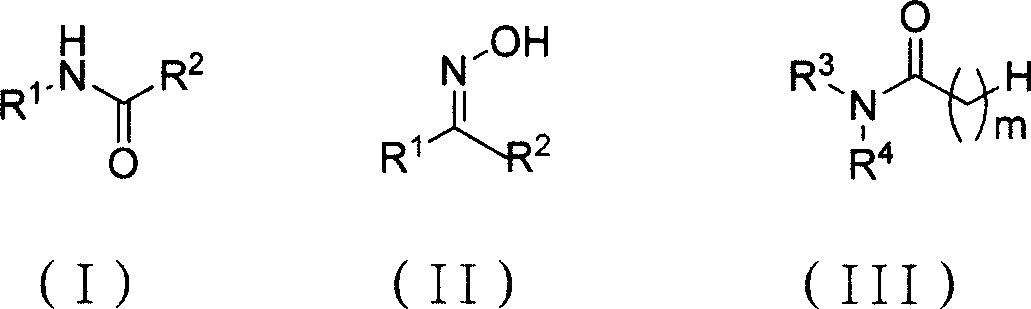
Chemical synthesis method for amide substance
A technology for chemical synthesis and amides, applied in the field of chemical synthesis of amides, can solve the problems of large acid consumption, easy deactivation of catalysts, and high reaction temperature, and achieves high reaction yield, low production cost, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The ratio of the amount of feed material to ketoxime: bis (trichloromethyl) carbonate: N, N-disubstituted amide is 3: 1: 3, ketoxime is acetophenone oxime, N, N-disubstituted amide is N, N-dimethylformamide, acetonitrile is used as a solvent, and its consumption is 6 times of the quality of bis(trichloromethyl)carbonate.
[0020] In a 150mL four-necked flask equipped with a thermometer, a reflux condenser and mechanical stirring, add 25mmol (7.4g) of bis(trichloromethyl)carbonate, dissolve it with 45g of acetonitrile, and add N,N-dicarbonate dropwise under an ice-water bath. Methylformamide 75mmol (5.5g), stirred at -5°C to 5°C for 1h, then added 75mmol (9.0g) of acetophenone oxime, heated to 85°C, and reacted at 80°C to 85°C for 2h, after the reaction was completed, Add saturated sodium carbonate solution to adjust to PH = 7, separate the organic layer, cool in an ice bath, stir for 10 minutes, filter, the filter cake is light yellow and it is crude acetanilide, after ...
Embodiment 2
[0022] The ratio of the amount of feed material to ketoxime: bis(trichloromethyl) carbonate: N, N-disubstituted amides is 3: 1.2: 3.6, and ketoxime is acetophenone oxime, and the feeding amount is 75mmol, and bis(trichloromethyl) Base) carbonate charging amount is 30mmol, N, N-disubstituted amide is N, and N-dimethylformamide charging amount is 90mmol, and acetonitrile is as organic solvent, and its consumption is 54g, i.e. two (trichloromethyl) carbonic acid 6 times the mass of the ester.
[0023] Other operations were the same as in Example 1, and the product yield of acetanilide was 90.8%, and the purity was 99.2%.
Embodiment 3
[0025] The ratio of the amount of feed material to ketoxime: bis(trichloromethyl)carbonate: N, N-disubstituted amides is 3: 1.5: 4.5, and ketoxime is acetophenone oxime, and the feeding amount is 75mmol, and bis(trichloromethyl) Base) carbonate charging amount is 37.5mmol, N, N-disubstituted amide is N, and N-dimethylformamide charging amount is 112.5mmol, and acetonitrile is as organic solvent, and its consumption is 67.5g, i.e. bis(trichloroform base) 6 times the mass of carbonate.
[0026] Other operations were the same as in Example 1, and the product yield of acetanilide was 91.1%, and the purity was 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com