Composition and method for inhibiting platelet aggregation
A compound, hydrate technology, applied in the field of mono- and dinucleoside polyphosphate compounds, which can solve problems such as high bleeding risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0301] Compound preparation
[0302] Those skilled in the art can easily synthesize the compounds of the present invention by well-known chemical methods. Mono-, di-, and triphosphate mononucleosides can be obtained from commercial sources or synthesized from nucleosides using various phosphorylation reactions found in the chemical literature. Nucleoside mono-, di-, or triphosphates can be activated by coupling agents such as, but not limited to, dicyclohexylcarbodiimide or 1,1'-carbonyldiimidazole, and then combined with another nucleoside mono-, di-, or triphosphate ( Can be the same (or different) condensation with the activated molecule to prepare symmetrical or asymmetrical dinucleoside polyphosphates. Activation of nucleoside triphosphates with dicyclohexylcarbodiimide yields cyclic trimetaphosphate as an activated species, which can advantageously react with various nucleophiles to add specific substituents to the terminal phosphate of the triphosphate superior.
[0...
Embodiment 1
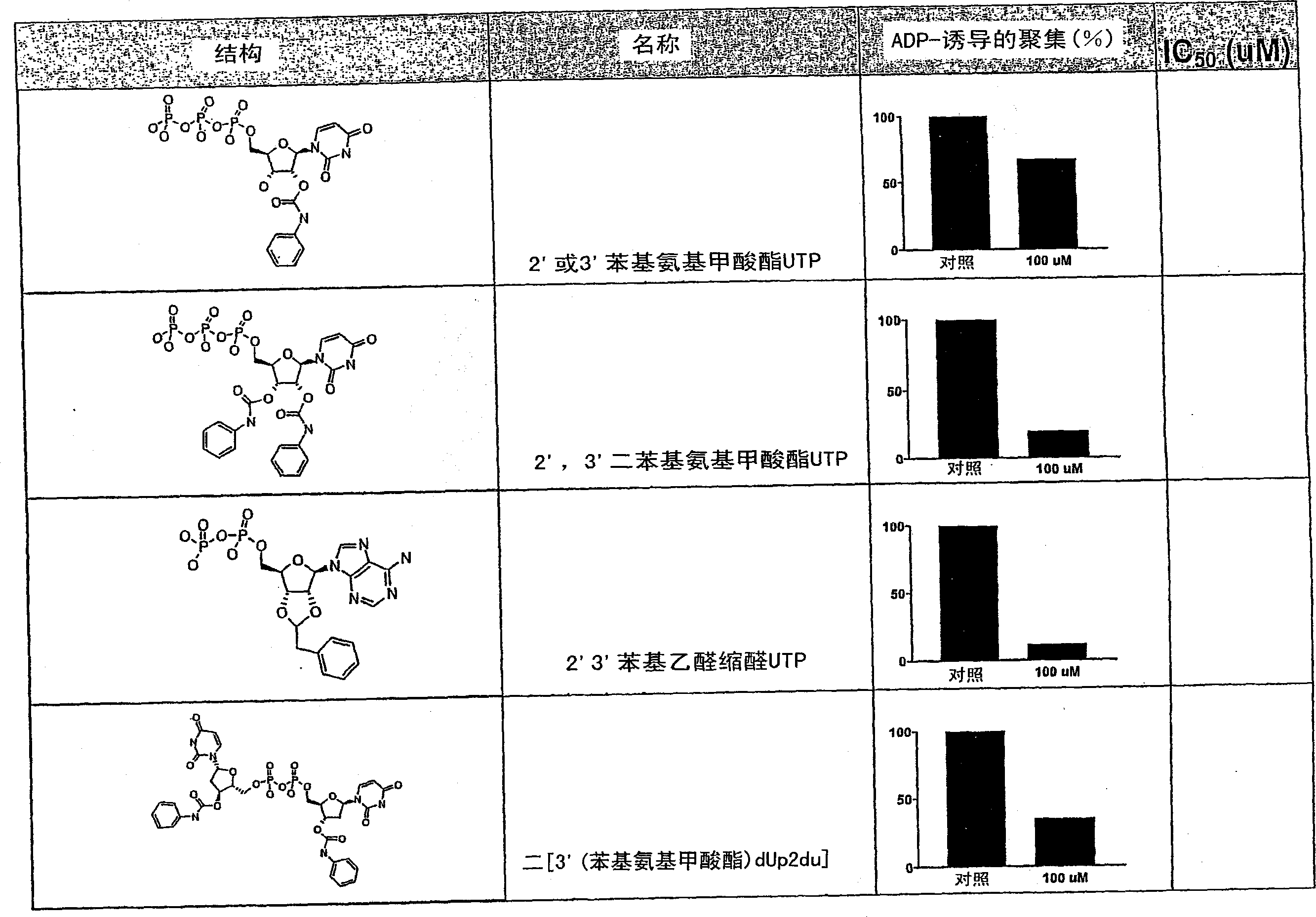
[0352] 2'(3')-O-((phenylaminocarbonyl)-uridine 5'-)triphosphate
[0353] Dissolve 5'-uridine ditributylammonium triphosphate (100mg, 0.176mmol; from trisodium salt by using Dowex 50Wx4H +Work up in water, then react the protonated material with excess tributylamine, strip and lyophilize), add phenylisocyanate (19 μL, 0.176 mmol). The reaction mixture was heated at 45°C for 15 minutes, at which time an additional portion of phenylisocyanate (19 μL, 0.176 mmol) was added. The solution was heated at 45°C overnight and DMF was removed on a rotary evaporator. The remaining oil was partitioned between water (2 mL) and ethyl acetate (2 mL), and the layers were separated. The aqueous layer was extracted two more times with ethyl acetate (2 ml each) and the water was removed on a rotary evaporator. The residue was dissolved in water (1.5ml), and the product was repeatedly injected into a preparative HPLC column (Alltech nucleotide / nucleoside C18, 7 microns, 10×250mm, gradient of 0.1...
Embodiment 2
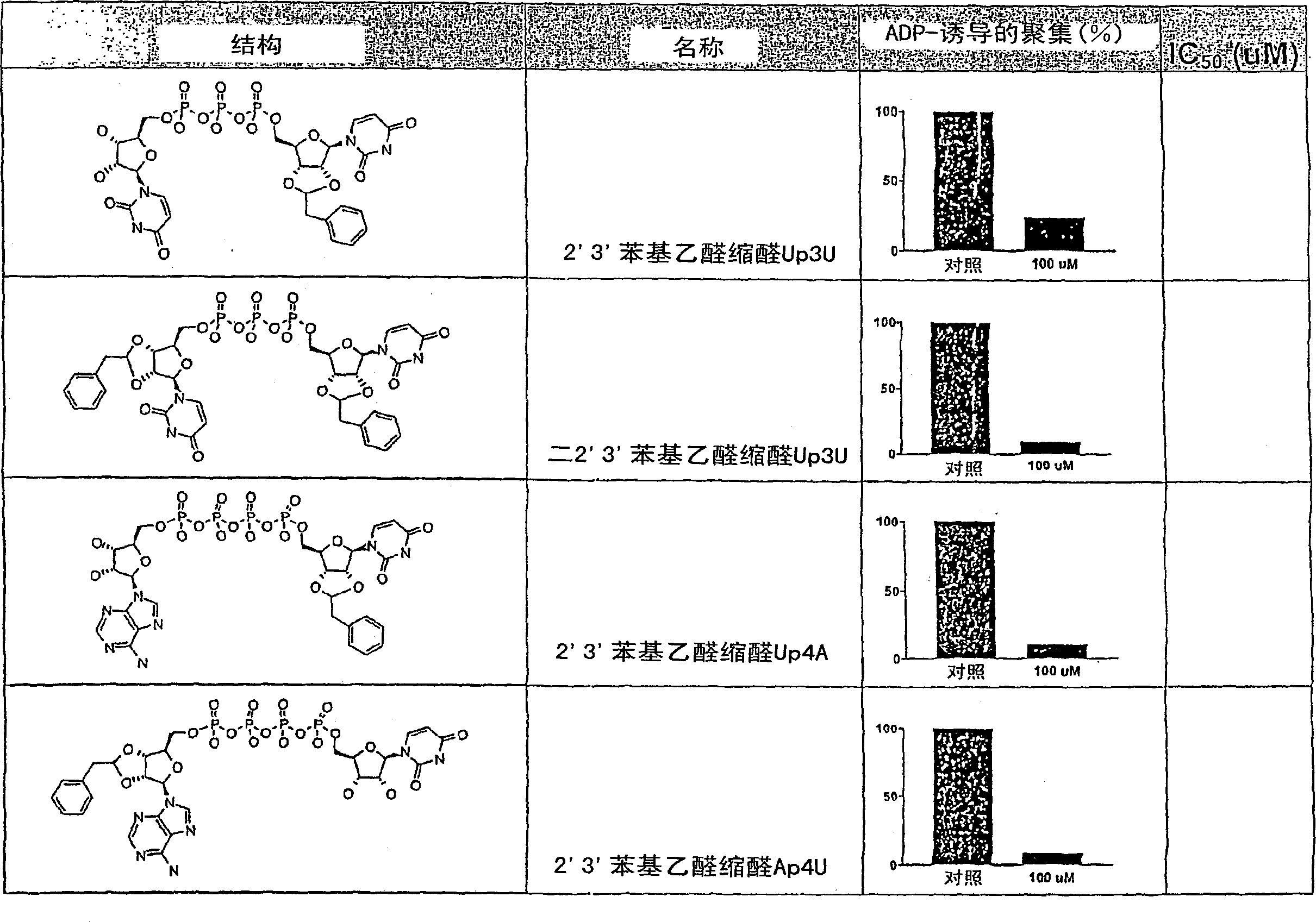
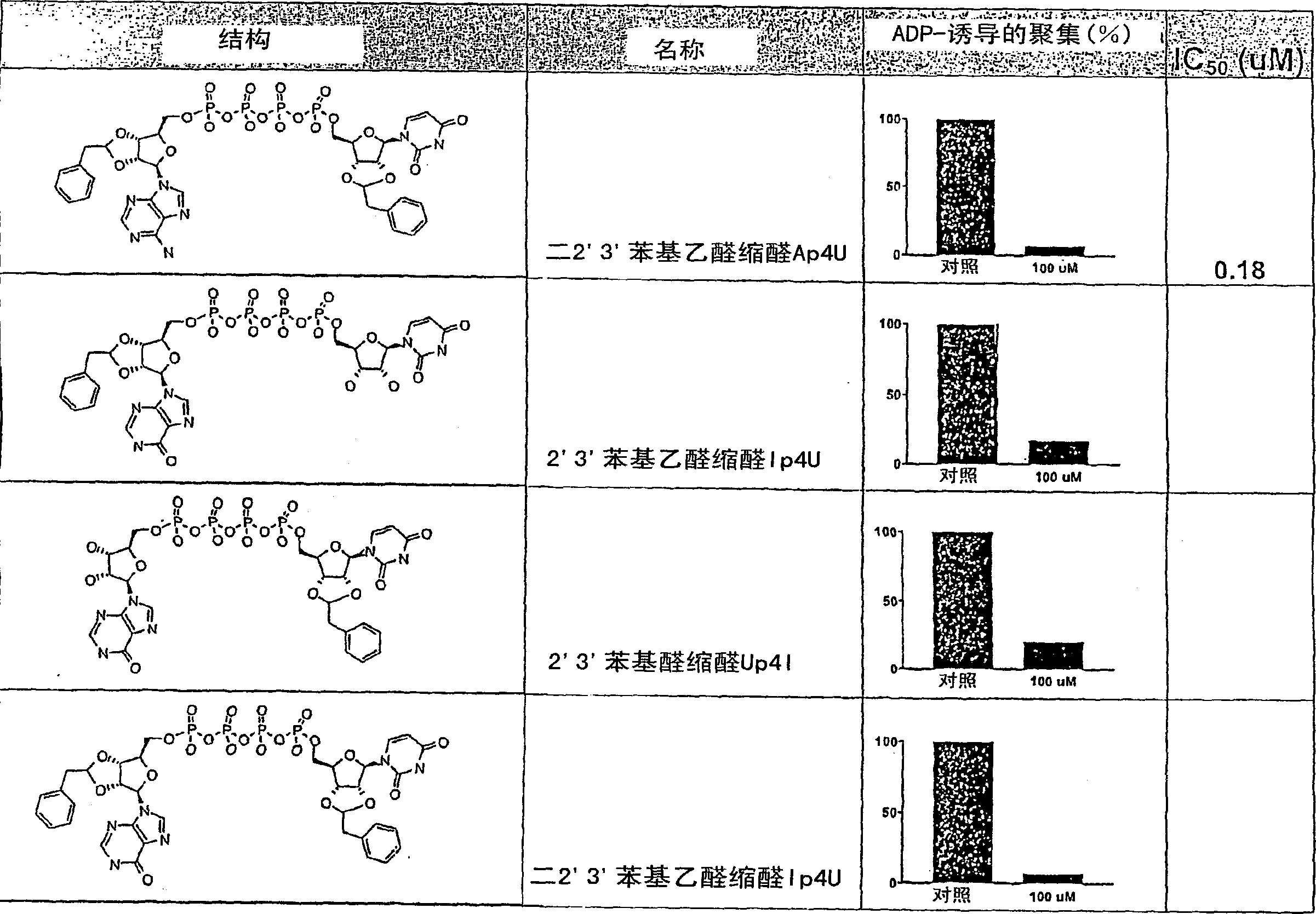
[0356] 2'(3')-O-(phenylaminocarbonyl)-P 1 , P 4 - bis(uridine 5′-)tetraphosphate ["monophenylcarbamate Up4U"], bis-2′(3′)-O-(phenylaminocarbonyl)-P 1 , P 4 - bis(uridine 5'-)tetraphosphate ["diphenylcarbamate Up4U"] and tris-2'(3')-O-(phenylaminocarbonyl)-P 1 , P 4 - Di(uridine 5′-)tetraphosphate ["triphenylcarbamate Up4U"]
[0357] Dissolve P in anhydrous DMF (2ml) 1 , P 4 - Di(uridine 5')'-tetraphosphate ditributyl modified salt (211 mg, 0.182 mmol; obtained from the tetrasodium salt by using Dowex 50Wx4H + , then reacted the protonated material with excess tributylamine, stripped and lyophilized), and added a portion of phenylisocyanate (40 μL, 3.64 mmol). The homogeneous reaction mixture was heated at 45°C overnight at which time TLC (silica gel, 50% isopropanol / 50% ammonium hydroxide) indicated substantial conversion to two products. The solvent was removed on a rotary evaporator and the residue was partitioned between water (7 mL) and ethyl acetate (10 mL). The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com