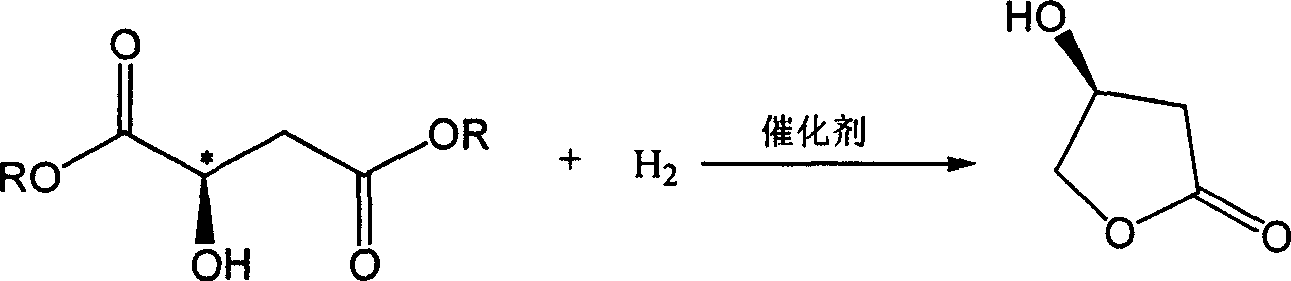
Method for synthetizing (S)3-hydroxy-gamma-butyrolactone by L-malate dioester catalytic hydrogenation
A malic acid diester, catalytic hydrogenation technology, applied in the direction of organic chemistry, etc., can solve the problems of difficult product purification, waste generation, and separation of butyrolactone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, the synthesis of catalyst:
[0018] 2.0 g of RhCl[Ph 2 PCH 2 CH 2 Si(OC 2 h 5 ) 3 ] 3 (Rh-P) complex, 50 mL of toluene and 10 g of SiO 2 Supported palladium catalyst (Pd-SiO 2 , Pd content is 10wt%) placed in the reaction flask, reflux reaction under nitrogen protection for 5 hours, filtered, the filter cake was washed three times with toluene, and dried to obtain the homogeneous-heterogeneous composite catalyst Rh-P / Pd-SiO 2 . By a similar method, respectively, with SiO 2 Supported nickel, platinum and ruthenium catalysts (Ni-SiO 2 , Pt-SiO 2 , Ru-SiO 2 ) instead of supported palladium catalyst Pd-SiO 2 , the composite catalyst Rh-P / Ni-SiO can be prepared 2 , Rh-P / Pt-SiO 2 and Rh-P / Ru-SiO 2 .
Embodiment 2~5
[0019] Embodiments 2 to 5, hydrogenation catalytic reaction: 0.5 gram of the composite catalyst obtained in Example 1, 10 gram of L-dimethyl malate and 50 milliliters of tetrahydrofuran were added in a pressure reactor, and after the reactor was replaced by hydrogen, the The mixture was stirred and reacted under 120 atmospheres of hydrogen at 100° C. for 24 hours. The catalyst was separated from the reaction mixture by filtration, and the filtrate was analyzed by chromatography. The results are shown in Table 1.
[0020] catalyst
Embodiment 6
[0025] Embodiment 6, the synthesis of catalyst:
[0026] 1.5 g of RhCl[Ph 2 PCH 2 CH 2 Si(OC 2 h 5 ) 3 ] 3 (Rh-P) complex, 50 mL of toluene and 10 g of SiO 2 Supported palladium catalyst (Pd-SiO 2 , Pd content is 5wt%) is placed in the reaction flask, reflux reaction under nitrogen protection for 5 hours, filtered, the filter cake is washed three times with toluene, and dried to obtain the homogeneous-heterogeneous composite catalyst Rh-P / Pd-SiO 2 . By a similar method, respectively, with SiO 2 Supported nickel, platinum and ruthenium catalysts (Ni-SiO 2 , Pt-SiO 2 , Ru-SiO 2 ) instead of supported palladium catalyst Pd-SiO 2 , the composite catalyst Rh-P / Ni-SiO can be prepared 2 , Rh-P / Pt-SiO 2 and Rh-P / Ru-SiO 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com