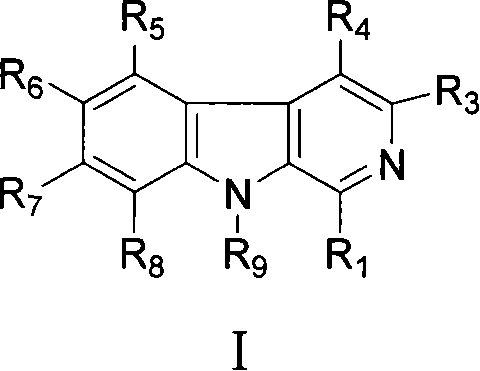
Flazin analog and preparation method and application thereof
A technology of analogs and drugs, applied in the field of drug synthesis, can solve the problem of no anti-tumor activity of Flazin analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
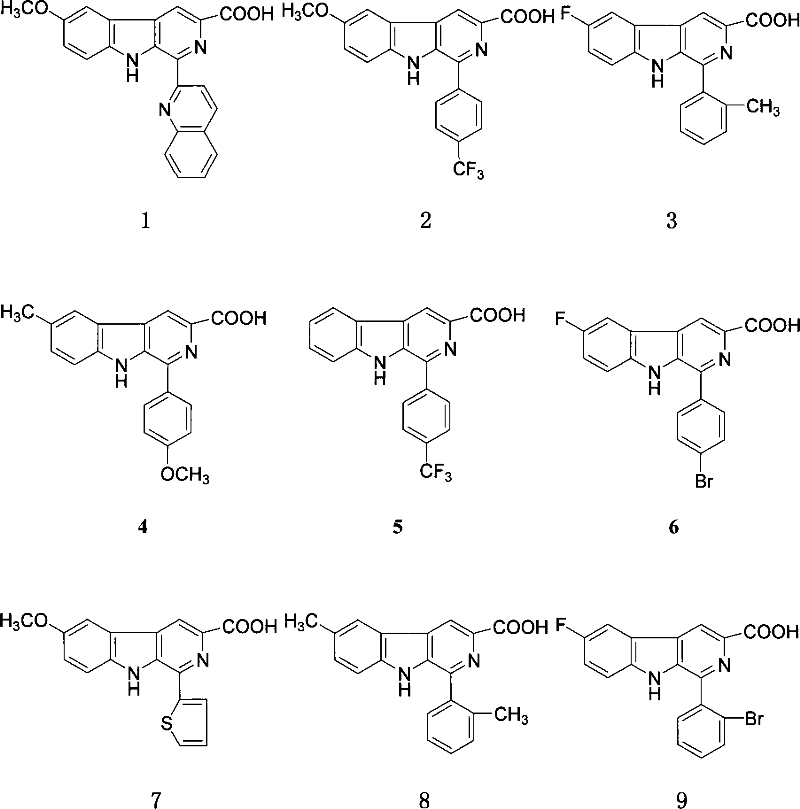
[0048] Example 1: Preparation of 1-(2'-quinoline)-6-methoxy-β-carboline-3-carboxylic acid (compound 1)
[0049] Step A: Synthesis of L-5-methoxy-tryptophan methyl ester
[0050] Add 100mL of dry methanol to 10mmol of L-5-methoxy-tryptophan, under magnetic stirring, cool to below -10°C, slowly add 10mL of thionyl chloride, then let it rise to room temperature naturally, and then heat to reflux for 3h . Spin off excess methanol and thionyl chloride under reduced pressure, then add an appropriate amount of water to dissolve, adjust the pH value to 9-10, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and obtain a white Solid 2.38g, Yield: 96%, mp 89-92°C, C 13 h 16 N 2 o 3 .FAB + -MS m / z: 249(M+1).
[0051] Step B: Synthesis of methyl 1-(2'-quinoline)-6-methoxy-β-carboline-3-carboxylate
[0052] Add 10 mL of dry dichloromethane to 1 mmol of L-5-methoxy-tryptophan methyl ester, 1 mmol ...
Embodiment 2
[0055] Example 2 Preparation of 1-(4'-trifluoromethylphenyl)-6-methoxy-β-carboline-3-carboxylic acid (compound 2)
[0056] Step A: Synthesis of 1-(4'-trifluoromethylphenyl)-6-methoxy-β-carboline-3-carboxylic acid methyl ester with L-5-methoxy-tryptophan methyl ester and 4'-trifluoromethylbenzaldehyde was used as raw material, and the operation was similar to step B of Example 1 to obtain 340.0 mg of light yellow solid, Yield: 85%, mp 165-168 °C, C 21 h 15 N 2 o 3 f 3 .FAB + -MS m / z: 401(M+1). 1 H NMR (400MHz, CDCl 3 ) δ (ppm) 8.89 (1H, s), 8.15 (2H, d, J = 8.0Hz), 7.84 (2H, d, J = 8.0Hz), 7.68 (1H, d, J = 2.4Hz), 7.55 ( 1H, d, J=8.8Hz), 7.27(1H, dd, J=2.4Hz, J=8.4Hz), 4.09(3H, s), 4.00(3H, s).
[0057] Step B: Synthesis of -(4'-trifluoromethylphenyl)-6-methoxy-β-carboline-3-carboxylic acid as 1-(4'-trifluoromethylphenyl)-6-methanol Oxy-β-carboline-3-carboxylic acid methyl ester was used as raw material, and the operation was similar to Example 1 step C to obtain 191.0...
Embodiment 3
[0058] Example 3 Preparation of 1-(2'-methylphenyl)-6-fluoro-β-carboline-3-carboxylic acid (compound 3)
[0059] Step A: Synthesis of L-5-fluoro-tryptophan methyl ester
[0060] Using L-5-fluoro-tryptophan as raw material, the operation is similar to step A of Example 1 to obtain 2.24 g of white solid L-5-fluoro-tryptophan methyl ester, Yield: 95%, mp 87-89 ° C, C 12 h 13 FN 2 o 2 .FAB + -MS m / z: 237(M+1).
[0061] Step B: Methyl 1-(2'-phenyl)-6-fluoro-β-carboline-3-carboxylate
[0062] Using L-5-fluoro-tryptophan methyl ester and 2-methylbenzaldehyde as raw materials, the operation is similar to step B of Example 1 to obtain 273.8 mg of yellow solid, Yield: 82%, 158-160 ° C, C 20 h 15 N 2 o 2 F FAB + -MS m / z: 335(M+1). 1 HNMR (500MHz, CDCl 3 )δ (ppm) 10.54 (1H, s), 8.72 (1H, s), 7.71 (1H, d, J=6.7Hz), 7.42 (2H, m), 7.25 (3H, m), 7.16 (1H, m ), 3.89(3H,s), 2.13(3H,s).
[0063] Step C: 1-(2'-phenyl)-6-fluoro-β-carboline-3-carboxylic acid
[0064] Using methyl 1-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com