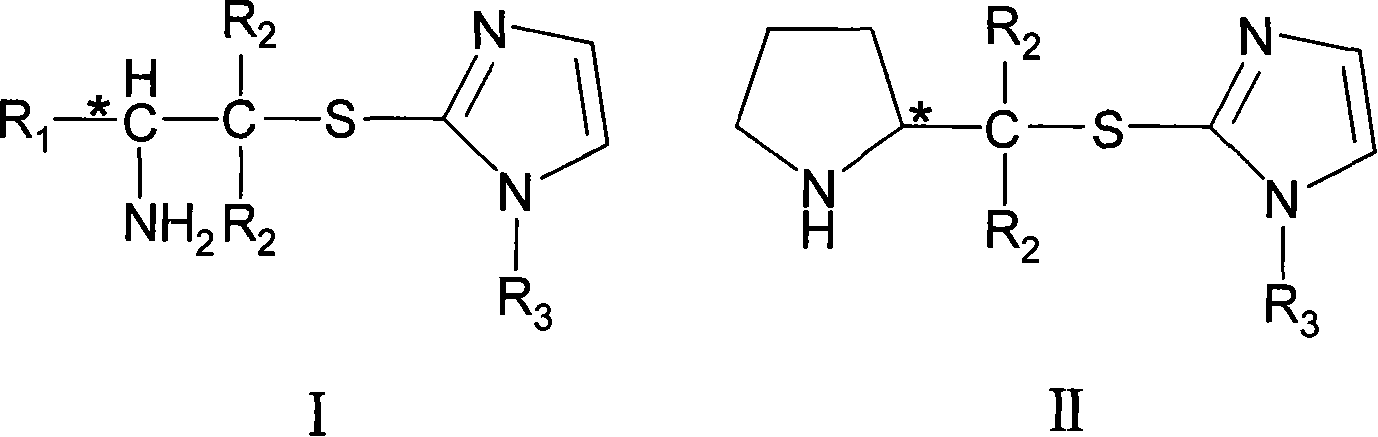
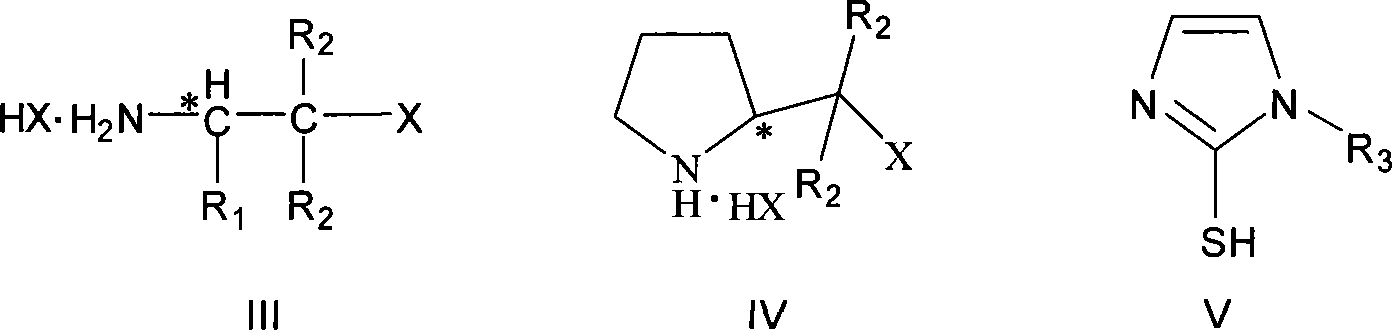
Chirality amine containing imidazole sulfur ether structure and preparation method and usage thereof
A technology of chiral amine and azole sulfide, applied in the application of chiral amine in asymmetric catalytic synthesis, the field of preparation of chiral amine, to achieve the effect of good chiral induction properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Preparation of 2-((S)-2-aminopropylthio)-1-hexylimidazole
[0041] Add (S)-1-(bromomethyl)ethylamine hydrobromide (21.90g, 0.1mol), 2-mercapto-1-hexylimidazole (18.41g, 98%, 0.1mol) and ethanol in a 100mL three-necked flask (60mL), reflux reaction for 12h, after neutralization, desolvation under reduced pressure, washed with ethyl acetate (2 × 20mL), after distillation to remove the solvent, column chromatography separation and purification to obtain the target compound (22.85g, yield 95% ), its specific rotation [α] D 20 =+32.1°.
Embodiment 2
[0042] Example 2: Preparation of 2-((S)-2-aminopropylthio)-1-methylimidazole
[0043] (S)-1-(bromomethyl)ethylamine hydrobromide (21.90g, 0.1mol), 2-mercapto-1-methylimidazole (18.41g, 98%, 0.1mol) and 1,2-dichloroethane (60mL), reflux reaction for 12h, after neutralization, desolvation under reduced pressure, washed with ethyl acetate (2 × 20mL), and then distilled to remove the solvent to obtain the target compound (16.20g, yield 95%), its specific rotation [α] D 20 =+33.2°.
Embodiment 3
[0044] Example 3: Preparation of 2-((S)-2-aminobutylthio)-1-ethylimidazole
[0045] Add (S)-1-(chloromethyl)propylamine hydrochloride (14.4g, 0.1mol), 2-mercapto-1-ethylimidazole (12.82g, 98%, 0.1mol) and acetonitrile ( 10mL), reflux reaction for 12h, after neutralization, desolventization under reduced pressure, washing with ethyl acetate (2 × 20mL), and distillation to remove the solvent, separation and purification by column chromatography to obtain the target compound (14.95g, yield 75%) , its specific rotation [α] D 20 =+33.5°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com