Benzothiazole azo compound, synthesizing method and usage thereof
A benzothiazole azo compound technology, applied in the field of benzothiazole azo compounds, can solve the problem of high temperature of ATRP polymerization, and achieve the effects of narrow molecular weight distribution, reduced energy consumption, and controllable molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
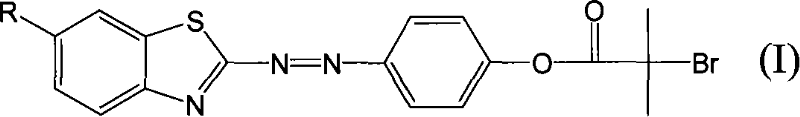
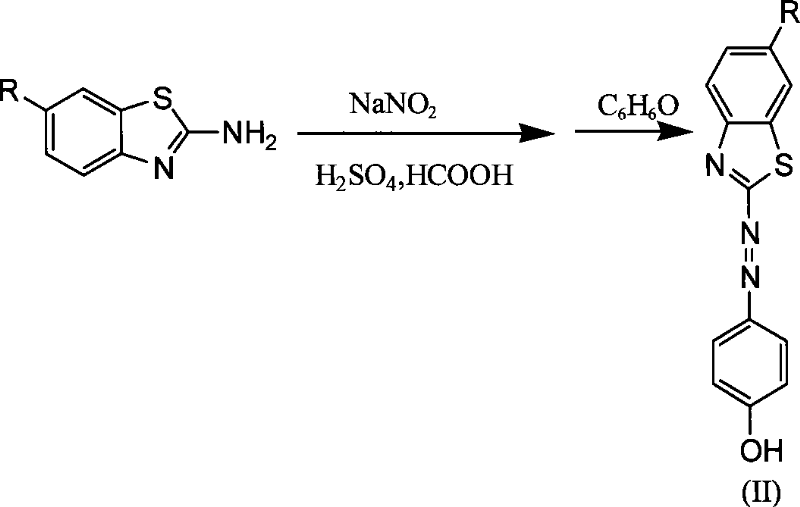
[0026] Take 1-3g of 2-aminobenzothiazole to make a sulfate solution, cool it in an ice bath, add 5-6g of 15-20% sodium nitrite aqueous solution dropwise, and react in an ice bath for 1 hour to obtain benzothiazole diazonium salt solution. Take 1 g of phenol, add 10 g of absolute ethanol and stir to dissolve, cool the ethanol solution of phenol to below 15°C, add the above diazonium salt solution dropwise, and react under ice bath for 24 hours. Filtration and washing with plenty of water yielded the benzothiazole azo intermediate.
[0027] Take 2-3g of benzothiazole azo intermediate, dissolve it in 40ml of tetrahydrofuran, add 1-3ml of triethylamine, and stir evenly under ice bath. Take 3-5g of bromoisobutyryl bromide and slowly add it dropwise to the above solution, react under ice bath for 1-3 hours, and then react at room temperature for 4-6 hours. The reaction solution was poured into water to precipitate and recrystallized to obtain the benzothiazole azo initiator.
[0...
Embodiment 2
[0030] Take 1-3g of 2-amino-6-nitrobenzothiazole to make a sulfate solution, cool it in an ice bath, add 5-6g of 15-20% sodium nitrite aqueous solution dropwise, and react in an ice bath for 1 hour to obtain 6- Nitrobenzothiazole diazonium salt solution. Take 1 g of phenol, add 10 g of absolute ethanol and stir to dissolve, cool the ethanol solution of phenol to below 15°C, add the above diazonium salt solution dropwise, and react under ice bath for 24 hours. Filtration and washing with plenty of water gave 6-nitrobenzothiazolyl azo intermediate.
[0031] Take 2-3g of 6-nitrobenzothiazole azo intermediate, dissolve it in 40ml of tetrahydrofuran, add 1-3ml of triethylamine, and stir evenly under ice bath. Take 3-5g of bromoisobutyryl bromide and slowly add it dropwise to the above solution, react under ice bath for 1-3 hours, and then react at room temperature for 4-6 hours. The reaction solution was poured into water to precipitate and recrystallized to obtain 6-nitrobenzoth...
Embodiment 3
[0034] Take 1-3g of 2-amino-6-methoxybenzothiazole to make a sulfate solution, cool it in an ice bath, add 5-6g of 15-20% sodium nitrite aqueous solution dropwise, and react in an ice bath for 1 hour to obtain 6 -Methoxybenzothiazole diazonium salt solution. Take 1 g of phenol, add 10 g of absolute ethanol and stir to dissolve, cool the ethanol solution of phenol to below 15°C, add the above diazonium salt solution dropwise, and react under ice bath for 24 hours. Filtration and washing with plenty of water gave 6-methoxybenzothiazolyl azo intermediate.
[0035] Take 2-3g of 6-methoxybenzothiazole azo intermediate, dissolve it in 40ml of tetrahydrofuran, add 1-3ml of triethylamine, and stir evenly under ice bath. Take 3-5g of bromoisobutyryl bromide and slowly add it dropwise to the above solution, react under ice bath for 1-3 hours, and then react at room temperature for 4-6 hours. The reaction solution was poured into water to precipitate and recrystallized to obtain 6-meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com