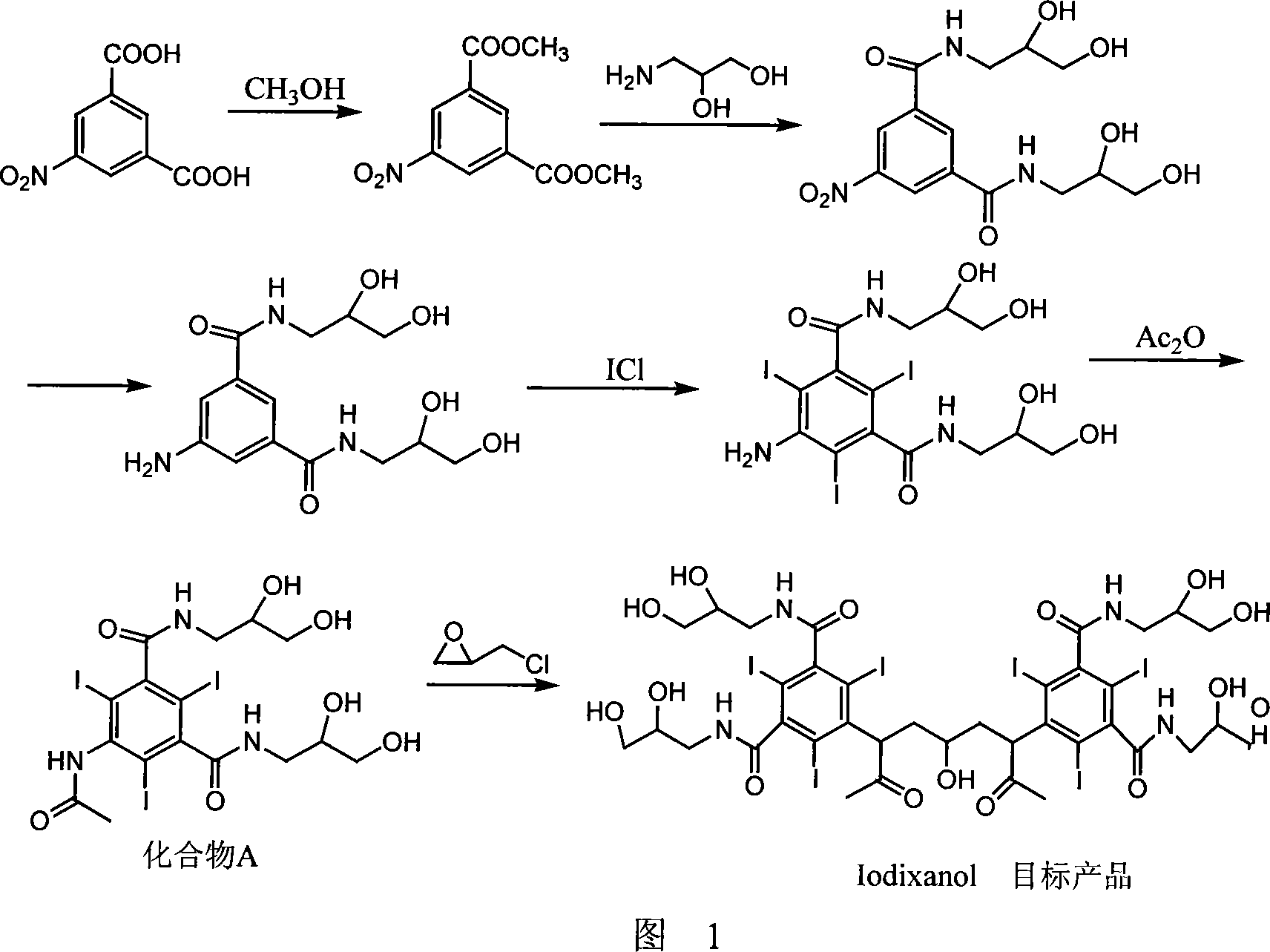
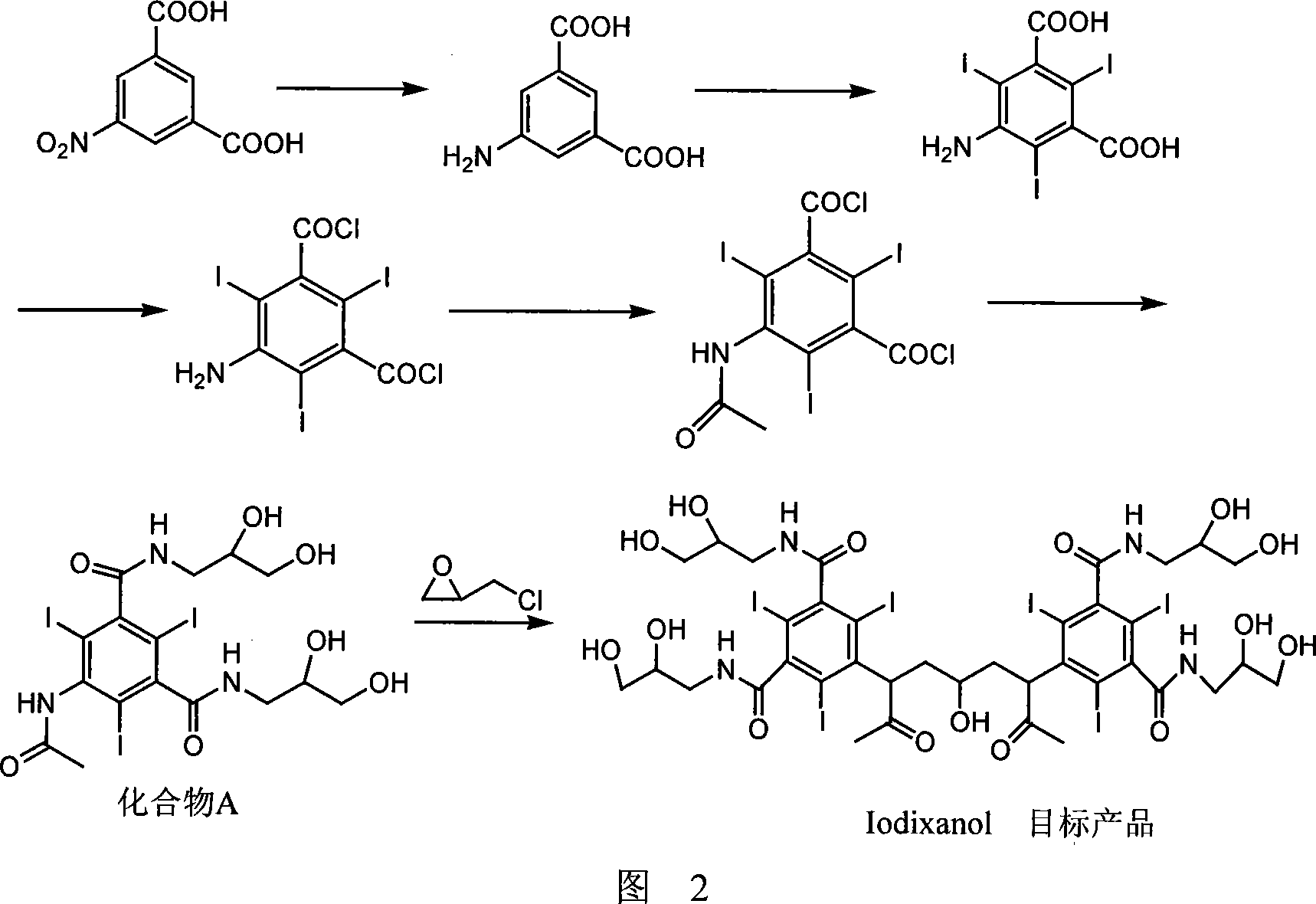
Preparation method of ioxaglic alcohol
A technology of iodixanol and triiodoisophthalamide is applied in the preparation field of iodixanol, can solve the problems of long reaction steps, high cost, etc., and achieves the advantages of easy operation, control and side reactions, and shortened reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0029] Embodiment 1-8, preparation of iodixanol
[0030] As shown in Table 1, take 0.6mol solid sodium hydroxide, dissolve it in 400ml solvent, heat up to 45°C, add 0.501mol compound 5-acetamido-N,N'-bis(2,3-dihydroxypropyl )-2,4,6-triiodoisophthalamide (hereinafter referred to as "compound A").
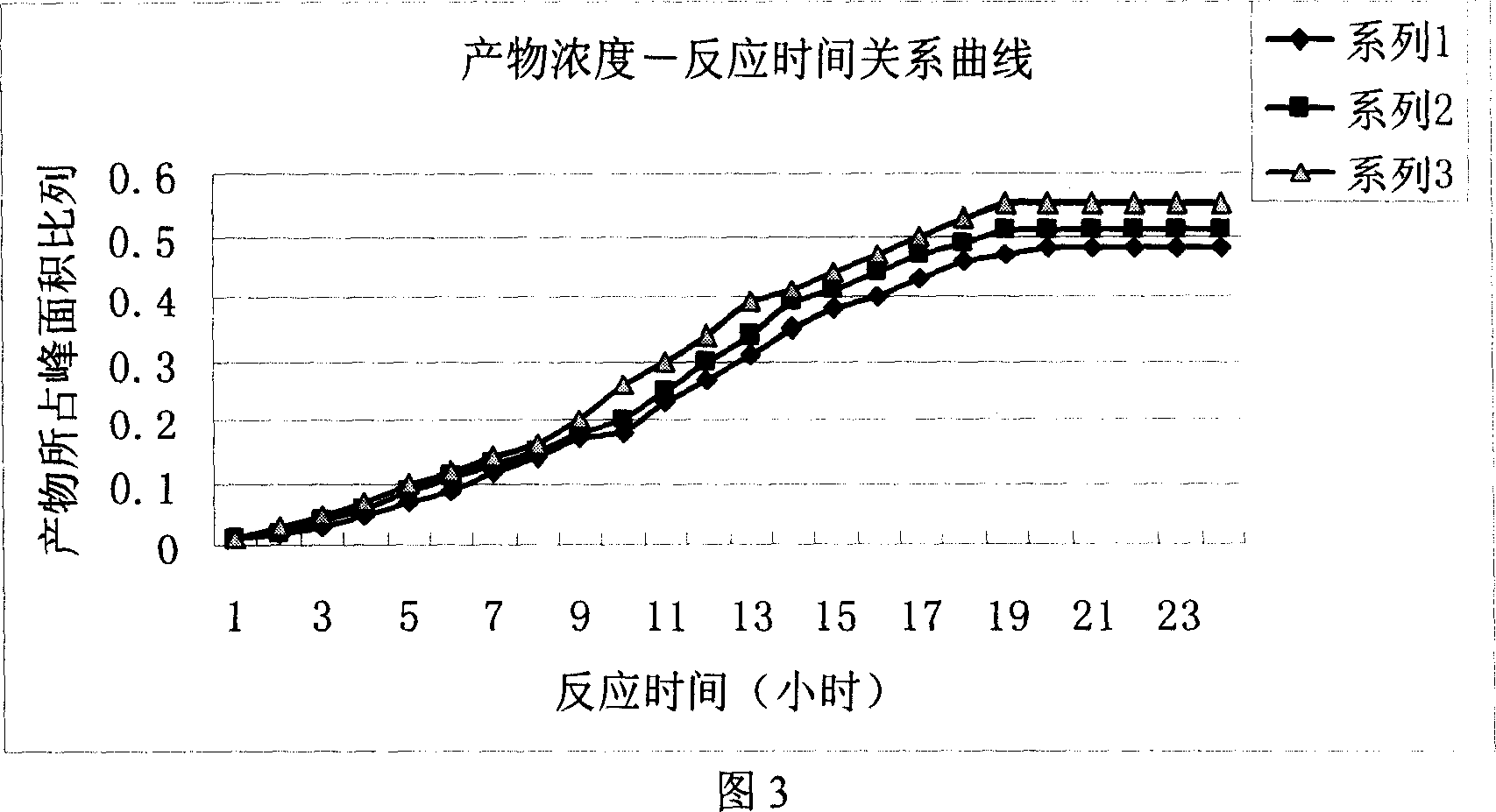
[0031] After all the solid raw materials are dissolved, the temperature is lowered to below 15°C, and 0.167mol of dimerization reagent containing the general structural formula (I) is added, and after stirring and reacting for 2 hours below 10°C, the temperature is raised to room temperature for reaction; A sample was taken every 2 hours, and the iodixanol content in the sample was detected by HPLC method, and the reaction time was plotted against the iodixanol content (Example 6), and the results were shown in Figure 3.
[0032] As can be seen from the results in Fig. 3, after about 18-20 hours of reaction, the content of iodixanol in the reaction mixture no longer increases signif...
Embodiment 9-20
[0035] Embodiment 9-20, preparation of iodixanol
[0036] As shown in Table 1, take 0.6 mol of solid sodium hydroxide, dissolve it in 400 ml of solvent, heat up to 45° C., and add 0.501 mol of compound A (recovered raw material + fresh raw material).
[0037] After all the solid raw materials are dissolved, the temperature is lowered to below 10°C, and 0.167mol of a dimerization reagent containing the general structural formula (II) is added, stirred and reacted at below 15°C for 2 hours, then warmed up to room temperature for reaction; A sample was taken every 2 hours, and the iodixanol content in the sample was detected by HPLC method, and the reaction time was plotted against the iodixanol content (Example 12, 17), and the results were shown in Figure 3.
[0038] As can be seen from the results in Fig. 3, after reacting for about 18-20 hours, the content of iodixanol in the reaction mixture no longer significantly increases, thus it can be determined that the reaction time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com