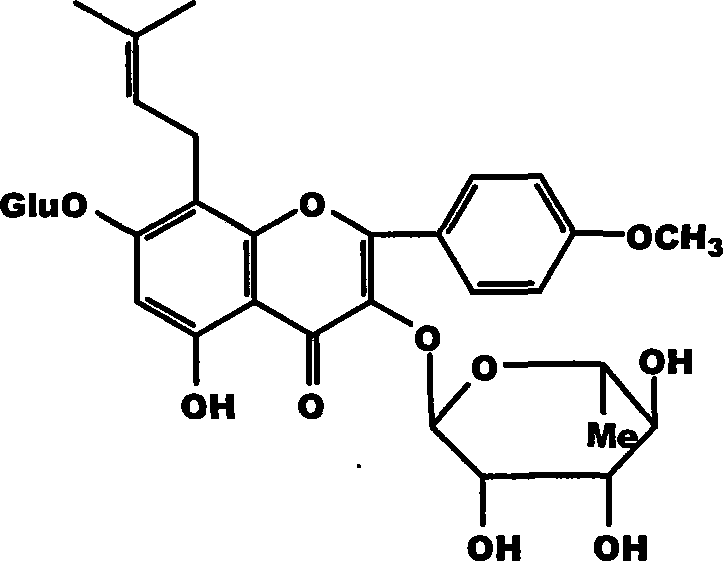
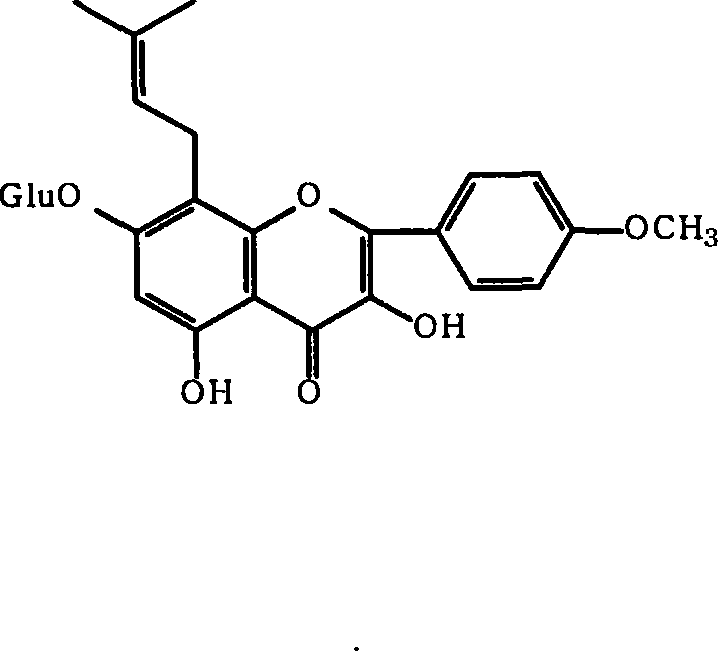
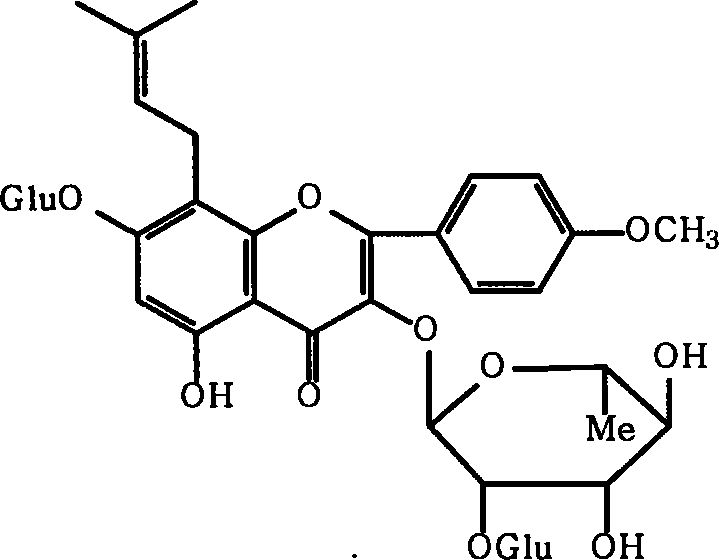
Medicinal composition containing epimedium active constituent and its application
A technology of epimedium extract and composition, applied in the direction of medical preparations containing active ingredients, drug combinations, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of Epimedium Extract
[0027] Epimedium leaf coarse powder is used 500g, extracts 3 times (60 ℃) with 3 liters of 60% ethanol solution, extraction time is 2 hours, filters and merges extraction liquid, reclaims organic solvent under reduced pressure, and aqueous solution adds macroporous adsorption resin (D101 or D140 column, wet weight 1000g), elute with 3 liters of water, then elute with 30%-85% ethanol solution, recover the ethanol eluate under reduced pressure, and dry to obtain 20g of part A extract (i.e. Epimedium extract of the present invention) , The yield is 3%, and the content of icariin in the extract of part A is determined to be 20%, and the total flavonoid content of Epimedium is 45.8%.
[0028] Take 400 g of the above Epimedium extract, repeat silica gel column chromatography, and perform chloroform:methanol gradient elution as usual (gradient elution refers to gradually increasing the methanol content in chloroform), thereby obtaining four c...
Embodiment 2
[0039] Preparation of Common Capsules of Epimedium Extract
[0040] Take Example 1 Epimedium extract 200g, microcrystalline cellulose (filler), sodium carboxymethyl starch (disintegrant) and pass through a 60-mesh sieve and mix evenly, add an appropriate amount of polysorbate 80 (wetting agent), Add 3% hydroxypropyl methylcellulose (E5 type is the binder) aqueous solution to prepare soft material in an appropriate amount, pass through a 20-mesh sieve and granulate. 40-50 ℃ oven blast drying. The dry granules are passed through a 20-mesh sieve for granulation, and the prescribed amount of talcum powder is added and mixed evenly. Fill No. 1 capsules according to the prescribed amount, and make 1000 capsules, each containing 200 mg of Epimedium extract. Usage: Three times a day, two capsules each time.
Embodiment 3
[0042] Preparation of Icariin Ordinary Tablets
[0043] Take 1 icariin, lactose (filler), and hydroxypropyl cellulose (disintegrating agent) of Example 1 and pass through a 60-mesh sieve and mix evenly, add an appropriate amount of polysorbate 80 (wetting agent), add 3% hydroxypropyl cellulose A proper amount of methylcellulose E5 (binder) aqueous solution is used to make soft materials, and granulated through a 20-mesh sieve. 40-50 ℃ oven blast drying. The dry granules are sieved through a 20-mesh sieve, added the prescribed amount of talcum powder, mixed evenly, and pressed into tablets to make 1000 granules, each containing 200 mg of icariin extract. Suggested taking method and dosage range: 4-8 capsules / day.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com