Methods for targeted delivery of genetic material to the liver
A technology for hepatocytes and mammals, which is applied in the fields of genetic material components, genetic material delivery pathways, and drug devices, and can solve problems such as damage to target organs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Catheter-Mediated Delivery of Adenoviral Gene Therapy Vectors to Rabbit Liver
[0084] For each experiment, New Zealand White rabbits (Millbrook Farms, Amherst, MA) each weighing approximately 4 kilograms were used. The adenoviral vector Ad2βgal used had a serotype 2 backbone with E1 deleted but E3 and E4 regions retained. The expression cassette consists of the cytomegalovirus (CMV) proximal early promoter and enhancer, the cDNA of nuclear localized β-galactosidase and the SV40 polyadenylation signal (Armentano, D. et al. (1997). J. Virol. 71: 2408-2416). Inject each rabbit with 1.5×10 12 Virus particles / kg (ratio of particles:infectious units=10:1) of Ad2[beta]gal virus.
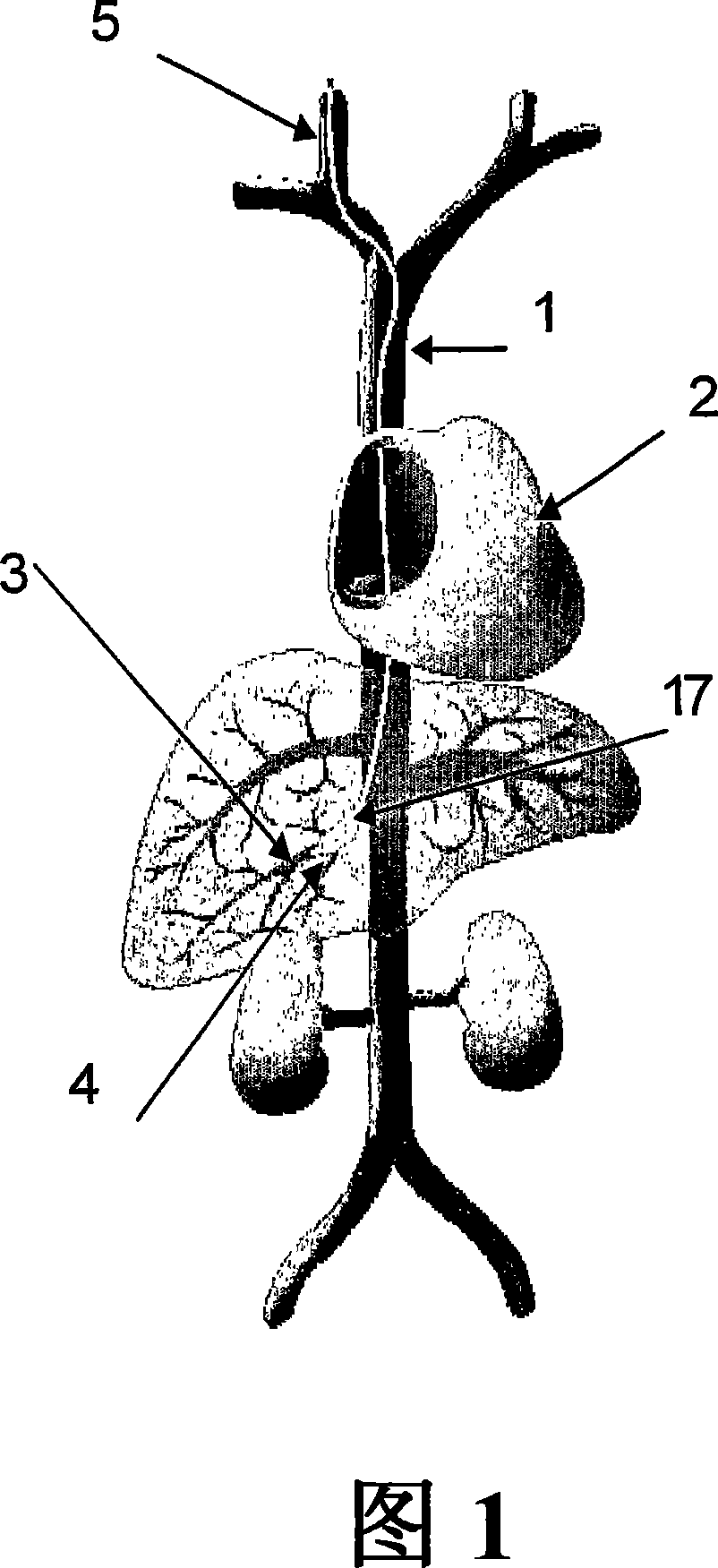
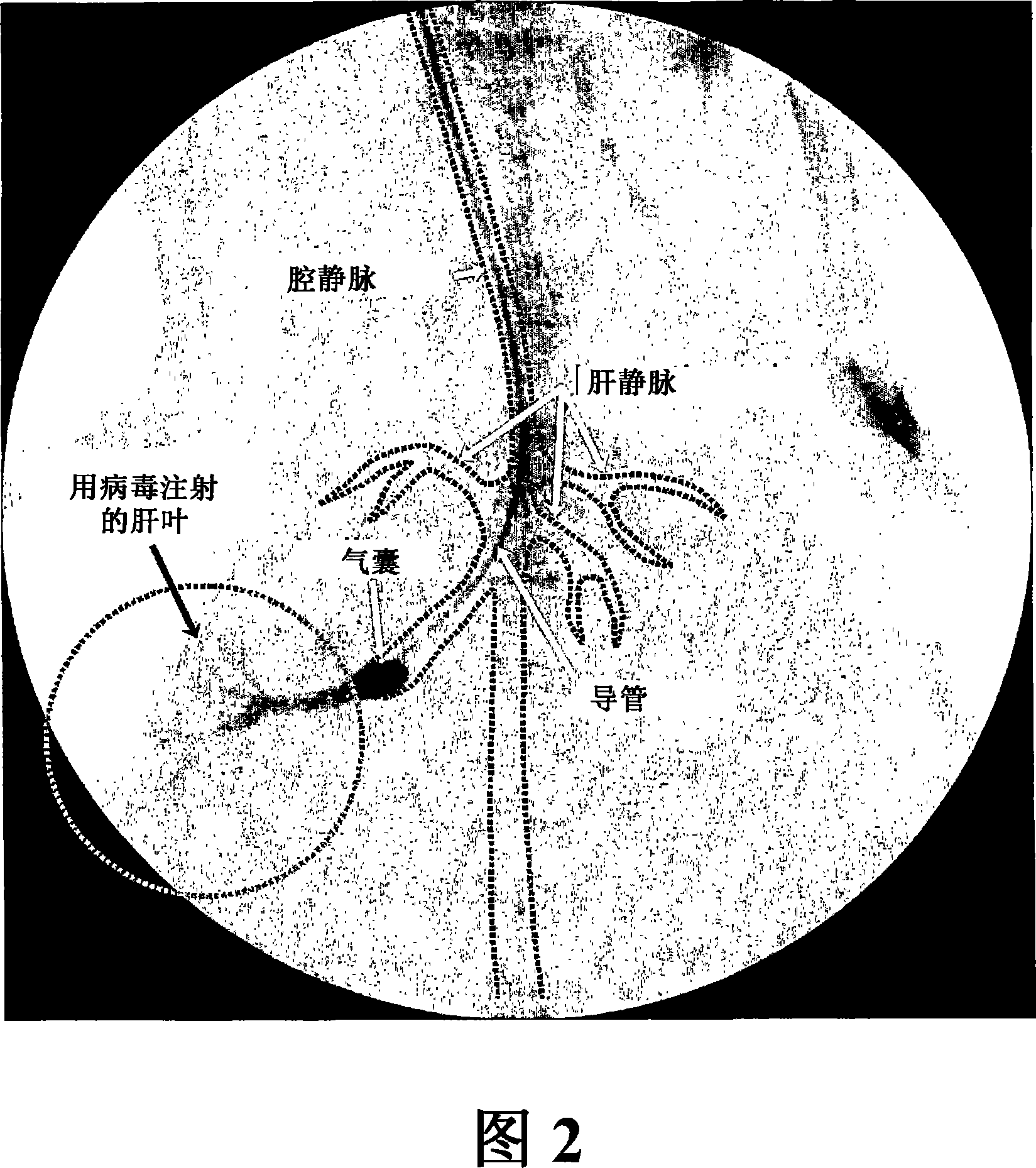
[0085] An adenoviral gene therapy vector was delivered to the liver of a rabbit using an embodiment of the method of the invention as follows. To access the rabbit's vasculature, the jugular vein was exposed by a midline incision starting from the mandibular arch and extending to the ...
Embodiment 2
[0100] Example 2: Comparison of Topical and Systemic Administration in Naive Rabbits
[0101] To determine whether local delivery of viral vectors conferred advantages over systemic delivery, Ad2βgal virus was delivered to rabbits by: 1) local delivery using balloon catheter-mediated delivery as described in Example 1, or 2) Systemic delivery using intravenous injection. does not depend on the delivery route, the 1.5 x 10 12 Virus particles / kg of Ad2βgal virus was injected into each rabbit.
[0102] Systemic delivery of viral vectors was performed according to the following protocol. The terminal ear vein of the sedated rabbit was accessed using an ear-safe 20-gauge catheter needle and wrapped with gauze and medical bandage. A luer-lock flush was attached to the catheter, and diphenhydramine; 1 mg / kg, was administered IV to control possible allergic reactions. Physiological saline in an amount of 8 ml containing Ad2βgal virus was injected into the ear vein at a rate of abo...
Embodiment 3
[0115] Example 3: Comparison of Topical and Systemic Administration in Passively Immunized Rabbits
[0116] Local, catheter-mediated delivery of adenoviral vectors was compared with systemic injection in animals with antiviral immunity to examine whether local injection confers advantages over systemic injection in this case. To this end, the experiment in Example 2 was repeated in rabbits passively immunized with stored human serum of known titre against adenovirus type 2 (anti-Ad2).
[0117] The day before virus administration, the terminal ear vein of sedated rabbits was accessed using an ear-safe 20-gauge vascular catheter needle and wrapped with gauze and medical bandages. A luer-lock flush was attached to the catheter, and diphenhydramine (1 mg / kg) was administered intravenously to control possible allergic reactions. Forty milliliters of stock human serum (Valley Biomedical, Winchester, VA) containing anti-Ad2 antibody was then injected at a rate of 0.1 ml / sec. This...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com