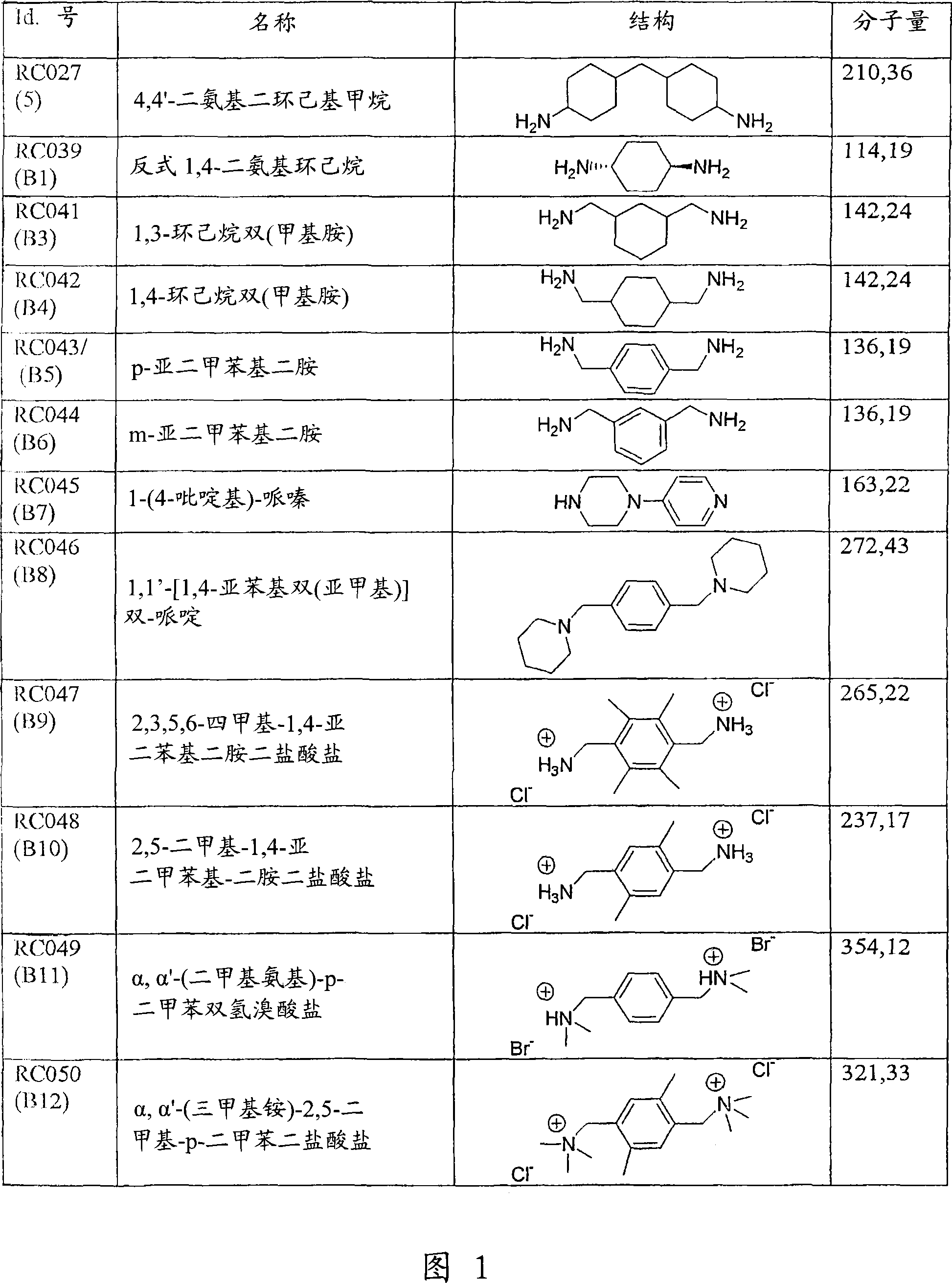
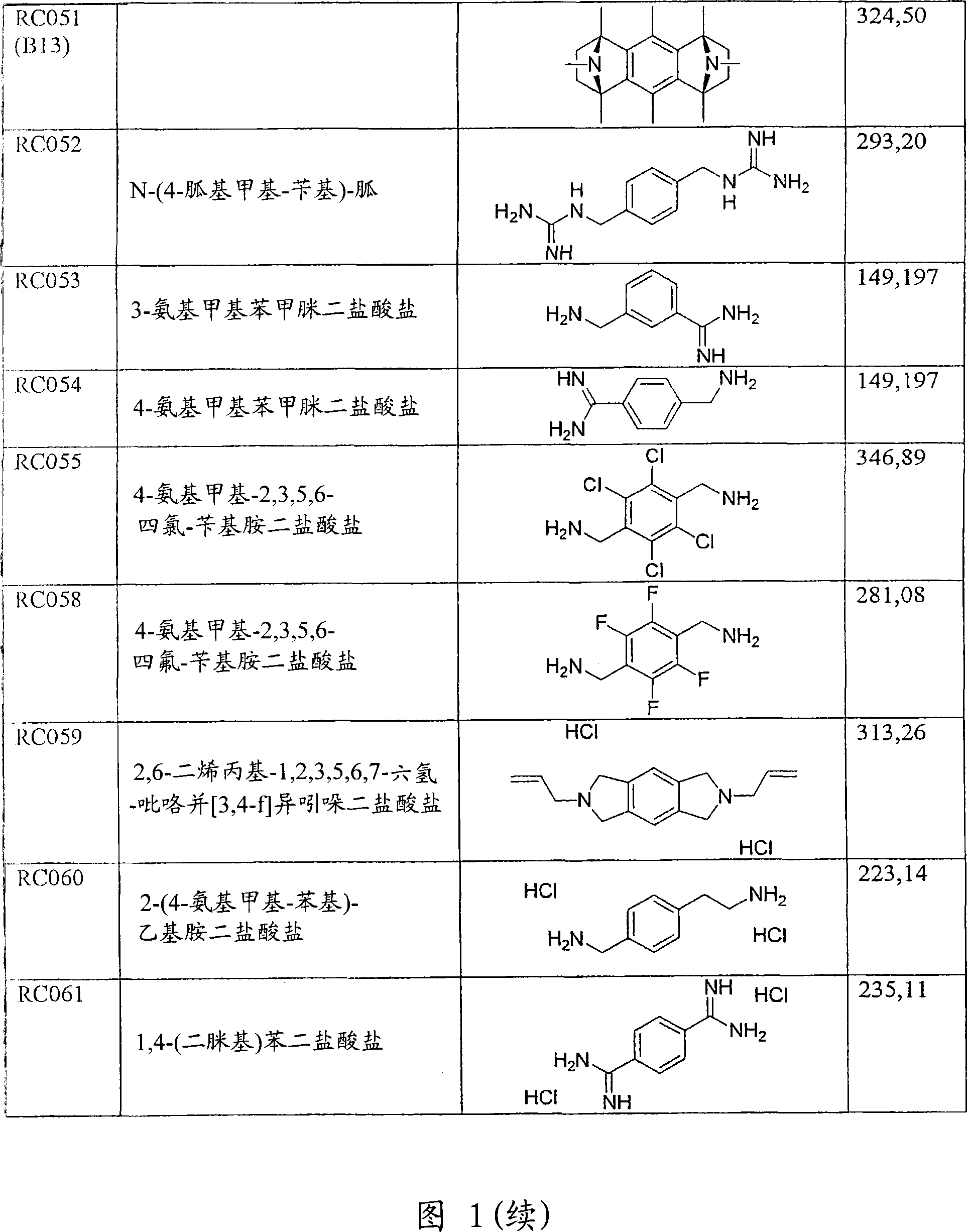
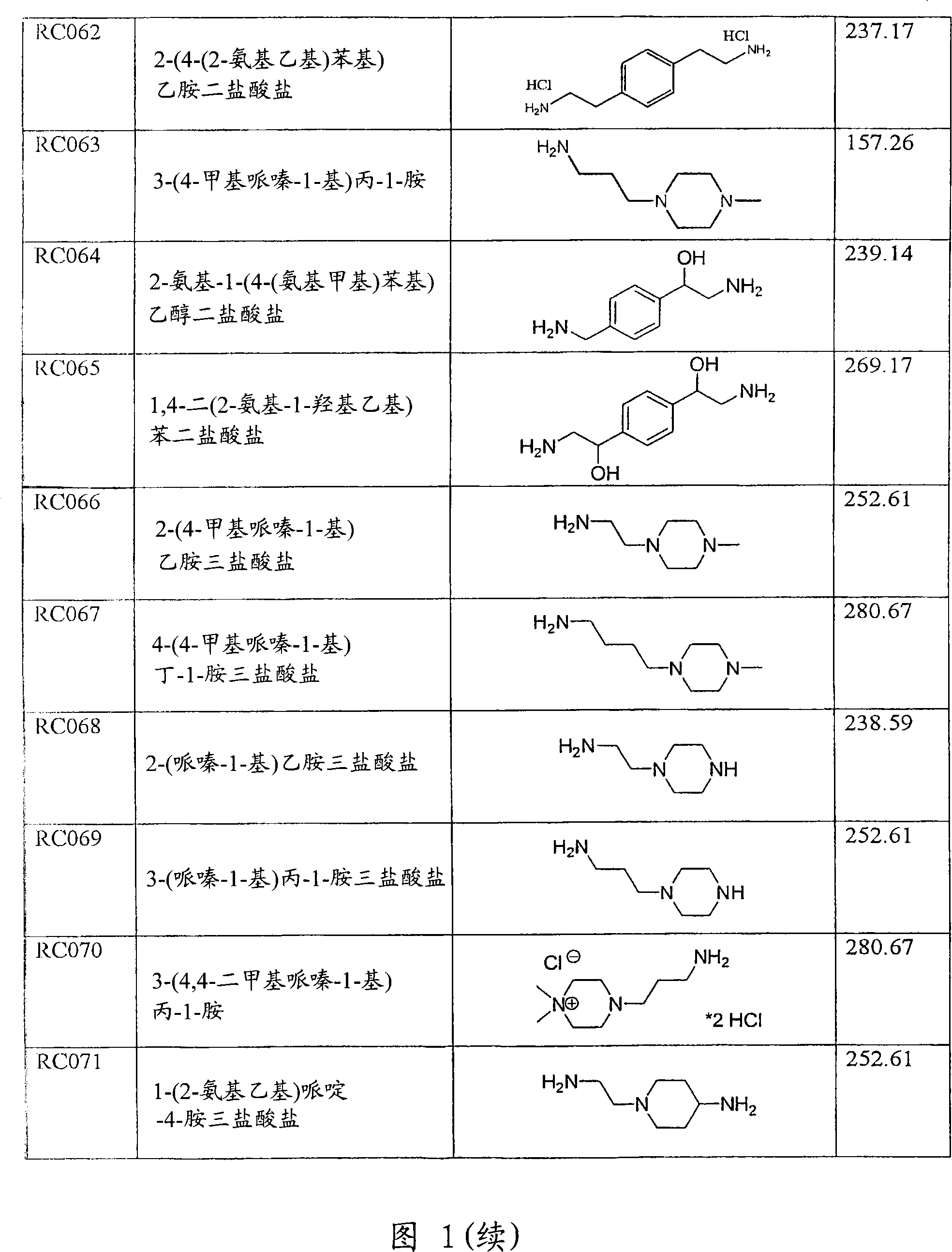
Use of compounds for the prevention of drug-induced cell toxicity
A compound and application technology, applied in the field of cytotoxic therapy, can solve the problems of reducing cytotoxicity, not disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0113] Preferred embodiment of X
[0114] In a preferred use embodiment of the present invention, X is an aromatic, carbocyclic, heterocyclic or heteroaromatic structure having 1-3 rings, each ring having 3-8 ring members and 0-3 heteroatoms, wherein each ring may be substituted at least once, wherein said substituent is selected from O, OH, phenyl, halogen, alkyl, alkenyl or alkynyl, substituted lower alkyl, substituted lower alkenyl or alkyne Base, aryl, heterocyclyl, heteroaryl, aryl-(C 1-4 )-Alkyl, Heteroaryl-(C 1-4 )-Alkyl, Heterocyclyl-(C 1-4 )-alkyl, cycloalkylalkyl, cycloalkyl, alkoxy, carboxy, trifluoromethyl, cyano, amino, nitro, O-alkyl, O-acyl, aminoalkyl and aminodioxane base.
[0115] In other more preferred embodiments, wherein X is an aromatic, carbocyclic, heterocyclic or heteroaromatic structure having 1-3 rings, with 5-6 ring members and 1 or 2 heteroatoms in each ring , wherein each ring may be substituted at least once, wherein said substituent is sel...
Embodiment 1
[0301] in vitro test
[0302] The inhibitory effect of p-xylylenediamine on the binding of gentamicin to immobilized megalin was assessed by proton surface resonance (SPR) analysis on a Biacore 2000 instrument. Megalin was purified from rabbit kidneys at 28-40 fmol / mm as described by Birn et al. 2 concentration is fixed. Sample dissolved in 10mM Hepes, 150mM NaCl, 1.5mM CaCl 2 , 1 mM EGTA, 0.005% Tween-20pH 7.4. The same buffer was used as running buffer. At time 0, the indicated concentrations of p-xylylenediamine (Figure 3) were applied to the chip and binding was expressed in relative response units (RU), i.e. the measured response between megalin and the control flow cell. difference. After 700 s, 1 mM gentamycin was injected together with buffer alone or various concentrations of p-xylylenediamine. Inhibition of gentamicin binding was scored using the difference in RU units obtained by injection of gentamicin alone and in the presence of antagonist. After each anal...
Embodiment 2
[0306] The in vivo effects of p-xylylenediamine (RC043 / B5) were tested. Gentamicin uptake in the mouse kidneys was measured after intraperitoneal (i.p.) administration of the compounds. Inject 50 μg / kg of tritiated gentamicin, using different concentrations of the inhibitor in each mouse. This corresponds to a clinical dose of gentamicin used in 1% of patients.
[0307] The results are shown in Figure 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com