Raw liquid for preparing chlorine dioxide, method and device for preparing chlorine dioxide
A chlorine dioxide and raw material solution technology, applied in the direction of chlorine oxidation, can solve the problems of low raw material conversion rate, low output, slow reaction speed, etc., achieve high raw material conversion rate, reduce acid discharge, and increase by-product value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
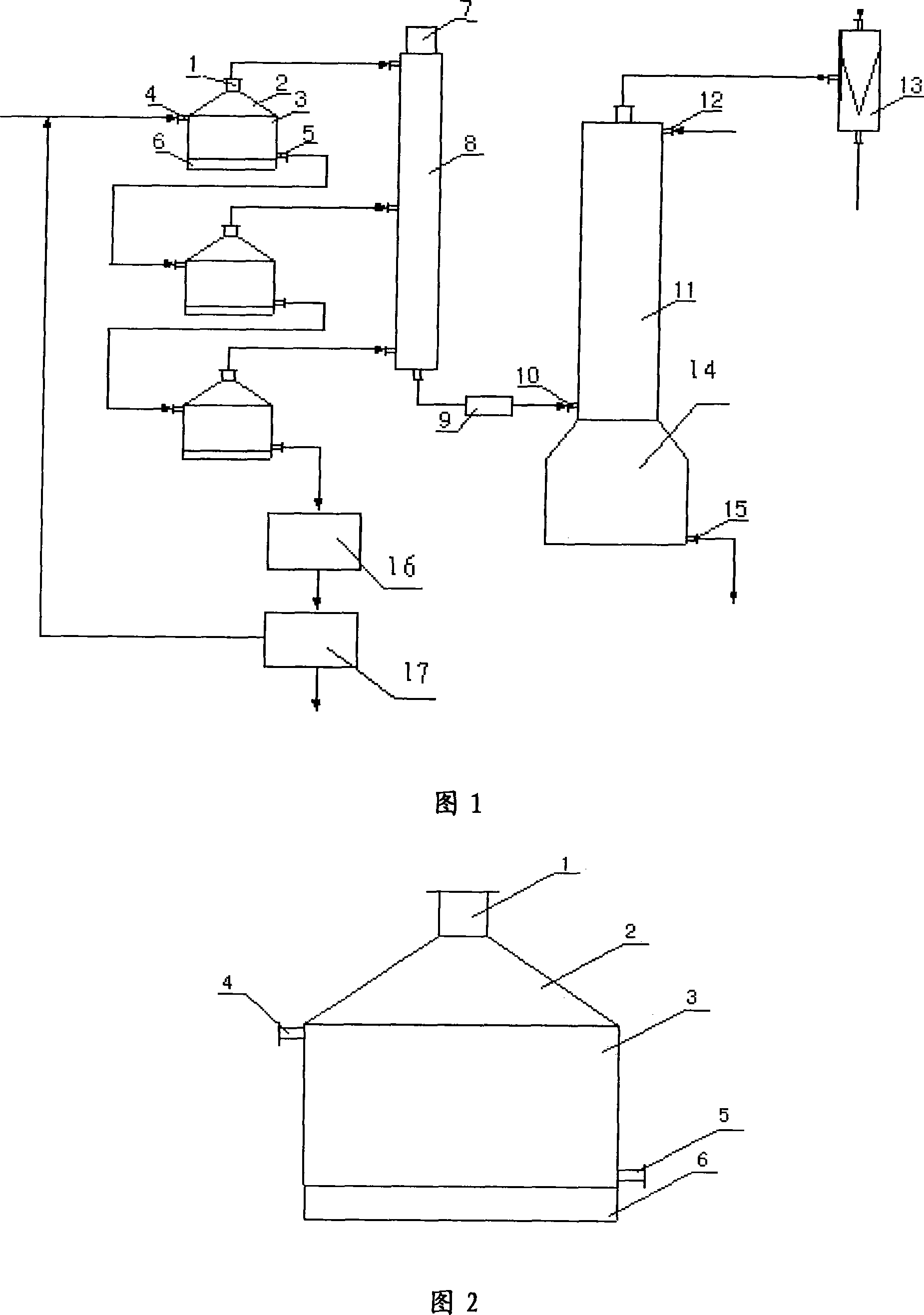
[0038] The method that embodiment 1, chlorate prepare chlorine dioxide, its reaction steps are:
[0039] 1. Configure the raw material solution: use water as a solvent, wherein the mass percentage of sodium chlorate is 20%, the mass percentage of urea is 2%, and the mass percentage of hydrogen peroxide is 1%;
[0040] 2. Feedstock solution and sulfuric acid are added to the first-stage reactor of the reaction device of the present invention at a uniform speed at a volume ratio of 1: 0.2, and the reaction temperature is 35° C., and the reaction is carried out under the condition that the negative pressure value in the reactor is 100~500mmHg. The generated chlorine dioxide is collected, and the residual liquid of the reaction is sent to the second-stage reactor;
[0041] 3. In the second-stage reactor, the reaction is carried out under the condition that the reaction temperature is 50°C and the negative pressure in the reactor is 100-500mmHg, and the generated chlorine dioxide i...
Embodiment 2
[0044] The method that embodiment 2, chlorate prepare chlorine dioxide, its processing step is:
[0045] 1, configuration raw material liquid: with water as solvent, wherein, the mass percentage of sodium chlorate is 33%, the mass percentage of urea is 3%, the mass percentage of sucrose is 2%;
[0046] 2. Feedstock solution and sulfuric acid are added to the first-stage reactor of the reaction device of the present invention at a uniform speed at a volume ratio of 1: 0.4, and the reaction temperature is 35° C., and the reaction is carried out under the condition that the negative pressure value in the reactor is 100 to 500 mmHg. The generated chlorine dioxide is collected, and the residual liquid of the reaction is sent to the second-stage reactor;
[0047] 3. In the second-stage reactor, the reaction is carried out under the condition that the reaction temperature is 45°C and the negative pressure in the reactor is 100-500mmHg, and the generated chlorine dioxide is collected,...
Embodiment 3
[0050] Embodiment 3, the method that chlorate prepares chlorine dioxide, its processing step is:
[0051] 1. Configure the raw material solution: use water as a solvent, wherein the mass percentage of sodium chlorate is 50%, the mass percentage of urea is 5%; the mass percentage of hydrogen peroxide is 3%;
[0052] 2. Feedstock solution and sulfuric acid are added to the first-stage reactor of the reaction device of the present invention at a uniform speed at a volume ratio of 1: 0.8, and the reaction temperature is 40° C. and the reaction is carried out under the condition that the negative pressure value in the reactor is 100 to 500 mmHg. The generated chlorine dioxide is collected, and the residual liquid of the reaction is sent to the second-stage reactor;
[0053] 3. In the second-stage reactor, the reaction is carried out under the condition that the reaction temperature is 55°C and the negative pressure in the reactor is 100-500mmHg, and the generated chlorine dioxide i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com