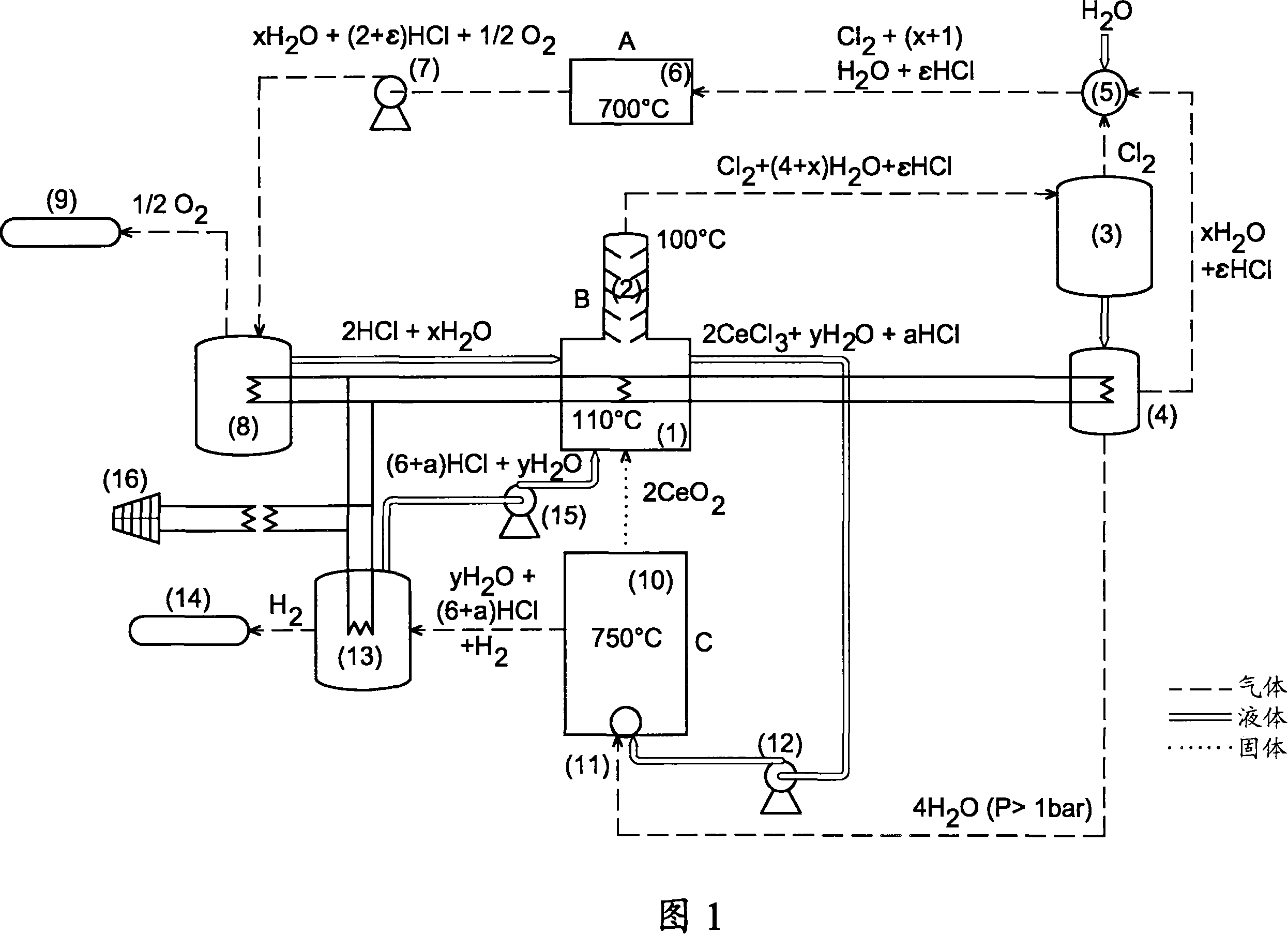
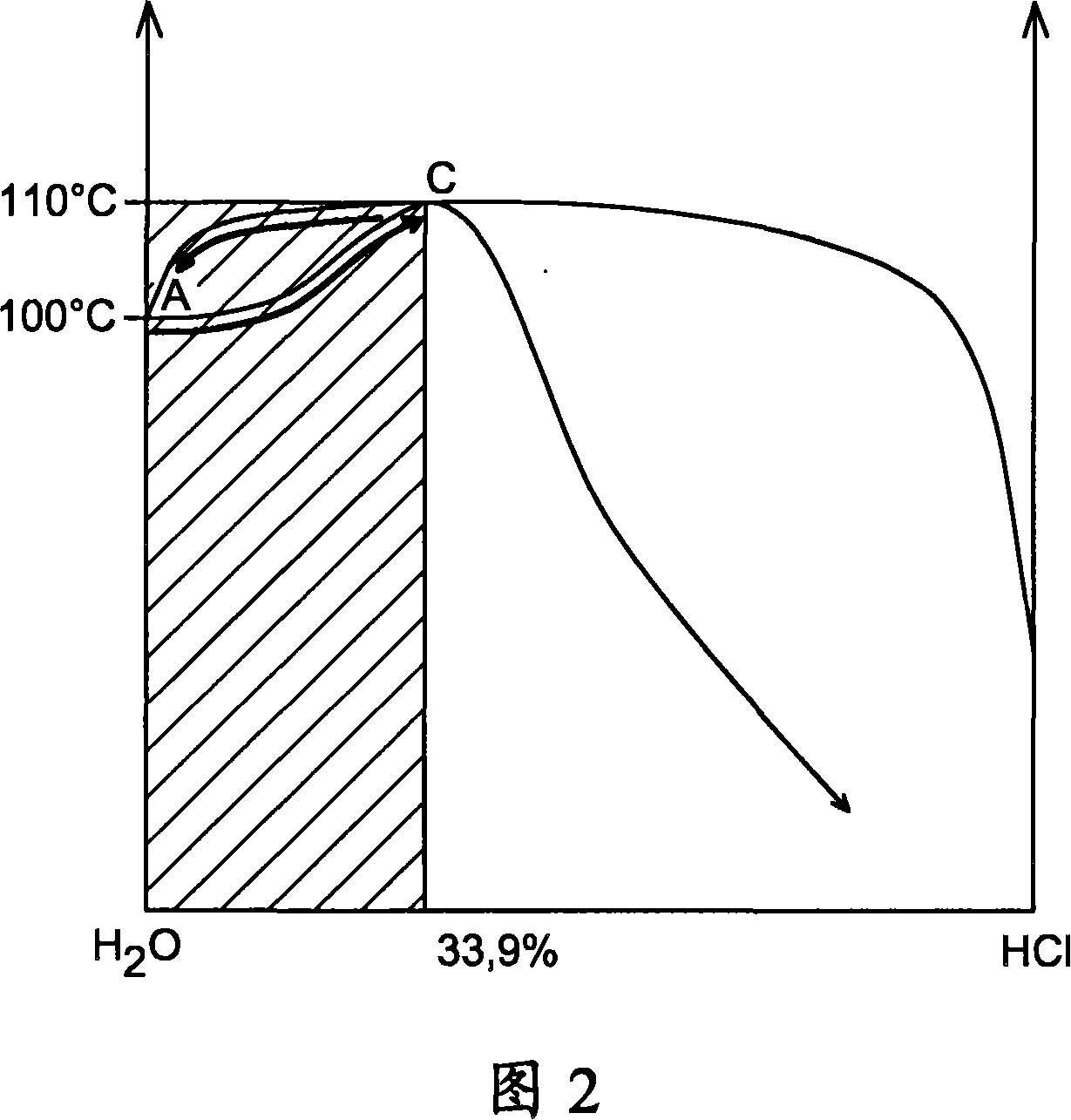
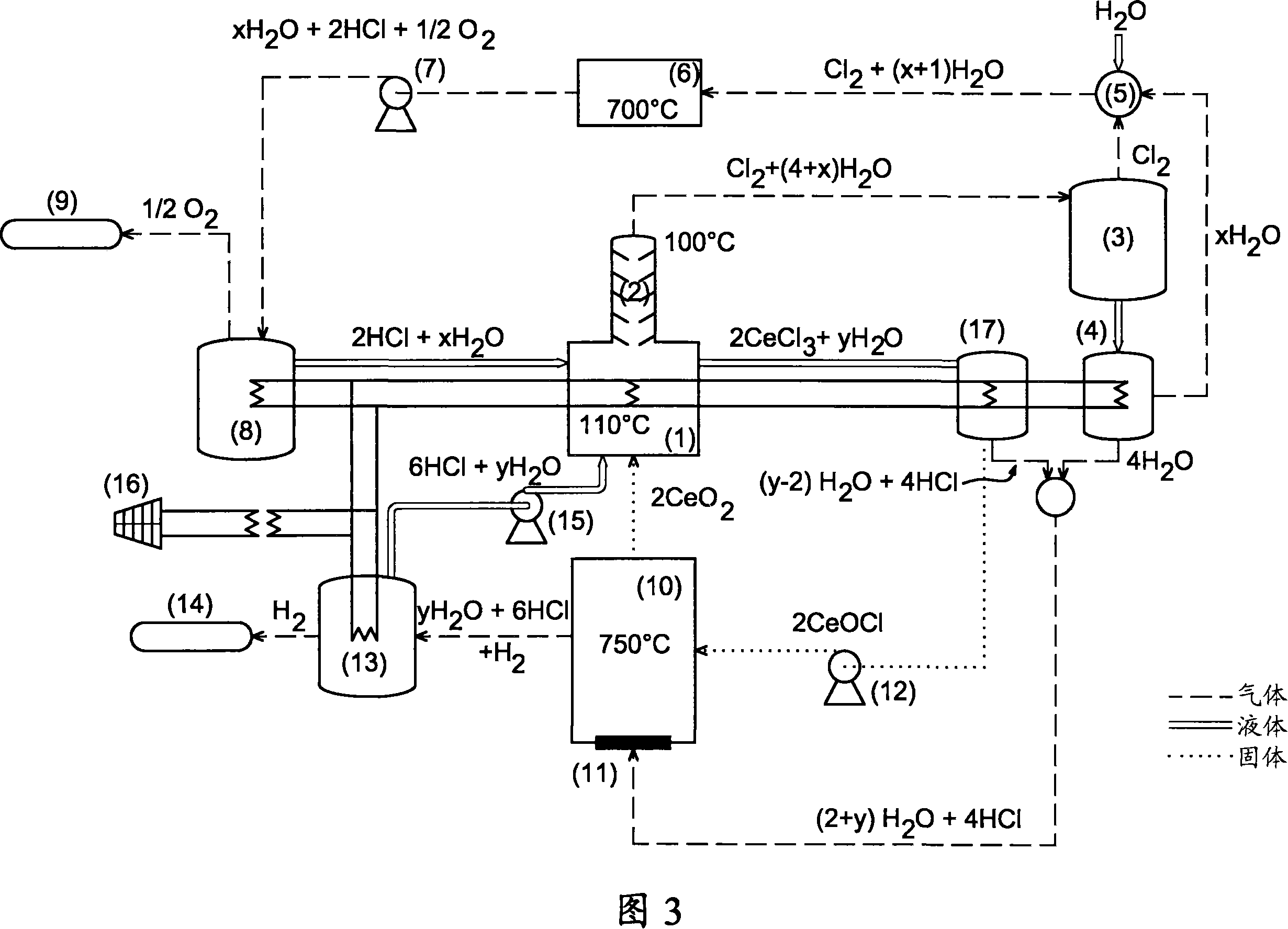
Method for producing hydrogen by thermochemical process based on hydrochlorination of cerium
A technology of thermochemistry and reaction, applied in chemical instruments and methods, inorganic chemistry, hydrogen production, etc., can solve problems such as difficulty in maintaining the stability of conversion reactors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] In this example, a test to implement reaction (B) was conducted.
[0143] These experiments consisted of making 47 ml of HCl azeotropic solution and 8 mg of CeO in a three-necked flask heated to 110°C with a reflux column on top. 2 (Equivalent to 1.5 times the stoichiometric amount required for total chlorination of the cerium) contact.
[0144] From this test, the possibility of reaching a reaction progress of 0.9 within 40 minutes can be confirmed.
[0145] In the process of using stoichiometric mixtures, it takes several hours to reach a progress of 0.9.
Embodiment 2
[0147] In this example, an experiment was conducted to realize the distillation related to the reaction (B).
[0148] These experiments consisted of making 31 ml of HCl azeotropic solution and 8 mg of CeO in a three-necked flask heated to 110°C with a Vigreux column on top. 2 (Equivalent to a stoichiometric mixture) contact so that all CeO 2 Reacts with chloride ions.
[0149] From this test, it has been confirmed that 95% of the water produced in the cerium oxide chloride process is recovered.
[0150] The pH of the recovered water later became 2, indicating that there was only a small amount of HCl in the solution (10 in the reactor). -2 mol.l -1 To 6.6mol.l -1 , That is, the ratio of 660), this should only slightly interfere with the reaction (A).
Embodiment 3
[0152] In this example, a test to implement reaction (B') was conducted.
[0153] These tests consisted of adding about 4g of CeCl in a round bottom flask heated to 150°C 3 Dissolve in 40 ml of water and allow the mixture to boil until it evaporates completely.
[0154] The recovered insoluble residue is equivalent to CeOCl.
[0155] The kinetics of the overall reaction is limited by the evaporation time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com