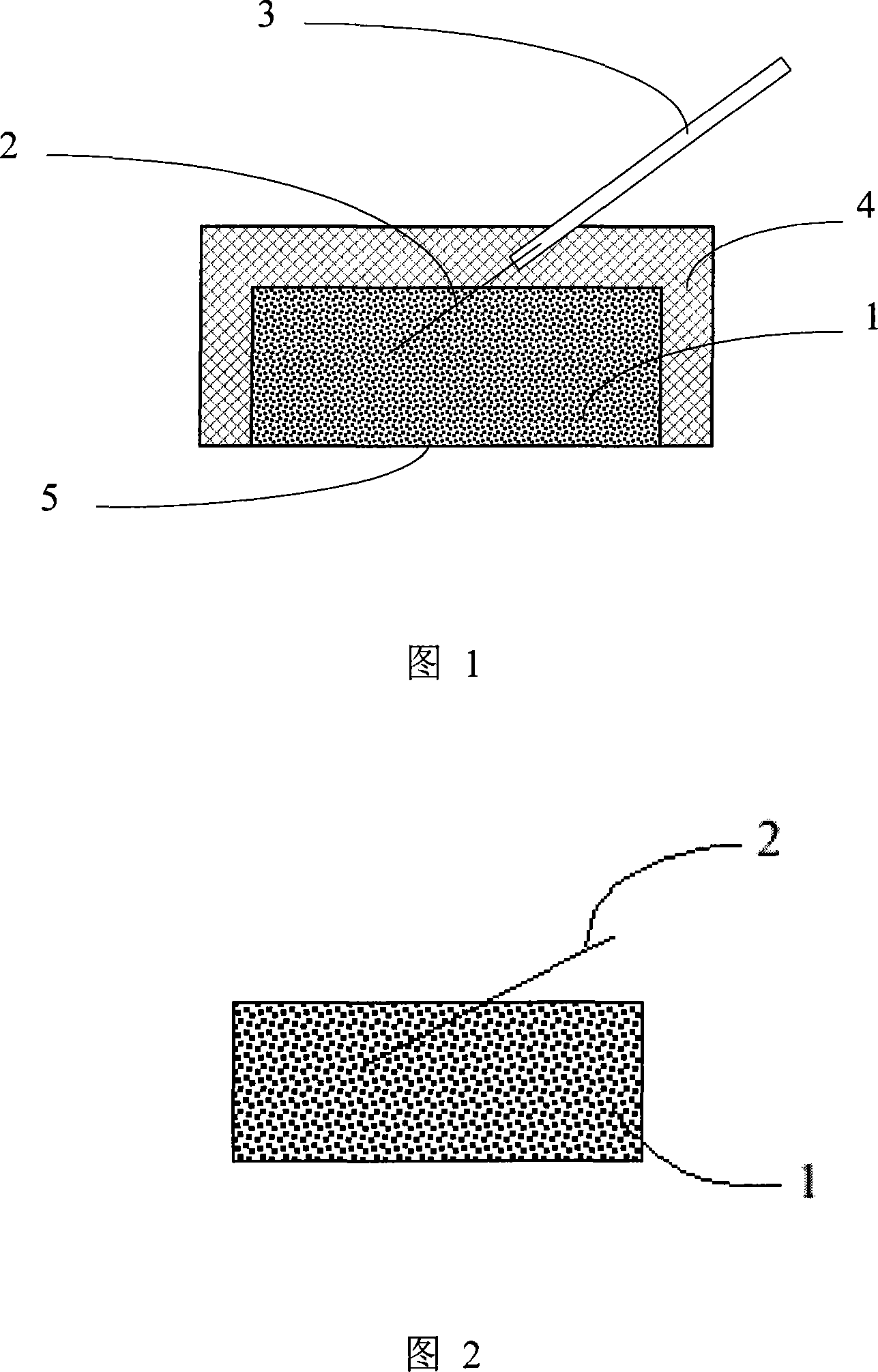
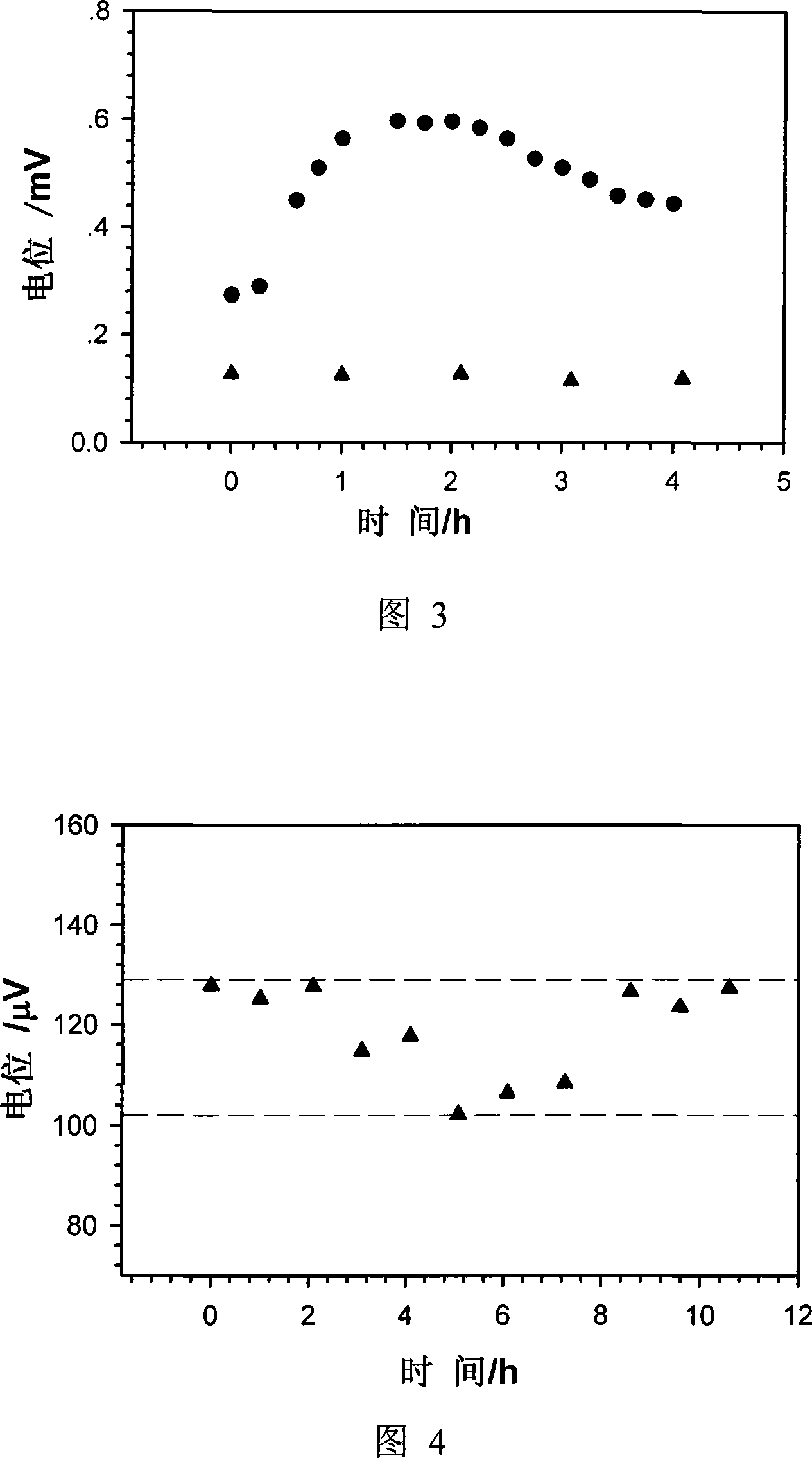
Silver/silver chloride powder solid electrode and preparation method
A solid electrode and silver chloride technology, which is applied in the field of biomedicine and electrochemical sensors, can solve the problem that the quality of silver chloride is difficult to control accurately and conveniently, and achieve high electrode potential stability, accurate and reliable detection, and strong anti-interference performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment one. Get the AgNO of 1.2g 3 (Analytical pure) was dissolved in distilled water to make a silver nitrate aqueous solution with a concentration of 0.02M, and 10g of Ag powder (average particle size 80μm) was added to the silver nitrate aqueous solution, stirred to disperse evenly to obtain Ag / AgNO 3 decentralized system. 0.4 g of NaCl (analytical pure) was formulated into a 0.1 M aqueous solution and added to the Ag / AgNO3 dispersion system under stirring. Stop stirring after the reaction is over, take the upper clear liquid and use silver nitrate and potassium chloride to detect whether there are excessive chloride ions and silver ions, if there are excessive ions, pour off the water layer and wash it with distilled water until there are no excessive reaction ions Appear. Finally, the water layer is poured off, and the reacted product is dried in an oven at a temperature of about 80°C. The ratio of the Ag / AgCl prepared in this way is about 1:0.1 in mass rati...
Embodiment 2
[0027] Embodiment two: get the AgNO of 3.5g 3 (Analytical pure) was dissolved in distilled water to make a silver nitrate solution with a concentration of about 0.5M, and 1g of Ag powder (average particle size of 1 μm, 99.95%) was dispersed in the silver nitrate solution system with a stirrer. Make the dispersion even. 1.1 g of NH 4 Cl (analytical pure) was made into a 0.2M solution and added dropwise to Ag / AgNO with a dropping funnel while stirring. 3 in a dispersed system. The following steps are the same as in Example 1. The ratio of Ag / AgCl thus prepared is approximately 1:3 by mass. Use 7500kg / cm of the prepared powder and fine silver wire in the mold 2 The pressure is pressed to form the electrode material 1 embedded with silver wire 2 as shown in Figure 2, and it is sintered in an electric furnace at 430° C. for 5 minutes. Then the silver wire 2 is welded to the wire 3 and packaged with epoxy resin 4 . The electrode was potentiostatically reduced (-0.1 V, SCE) in...
Embodiment 3
[0028] Embodiment three. Get the AgNO of 2.4g 3 (Analytical pure) was dissolved in distilled water to form a silver nitrate aqueous solution with a concentration of 0.05M, and 1g of Ag powder (average particle size of 1 μm, 99.95%) was dispersed in the silver nitrate solution system with a stirrer. Make the dispersion even. 1.05 g of KCl (analytical pure) was made into a KCl aqueous solution with a concentration of 0.3 M, and was added into the Ag / AgNO3 dispersion system with a dropping funnel while stirring. The following steps are the same as in Example 1. The ratio of Ag / AgCl prepared in this way is about 1:2 in mass ratio and 1:1.5 in converted molar ratio. Use 4500kg / cm2 The pressure is pressed to form the electrode material 1 embedded with silver wire 2 as shown in Figure 2, and it is sintered in an electric furnace at 250° C. for 15 minutes. Then the silver wire 2 is welded to the wire 3 and packaged with epoxy resin 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com