Tetraphenylethylene-based polypyrazole nitrogen-containing heterocyclic compound as well as preparation method and application thereof
A nitrogen heterocyclic compound and tetraphenylethylene technology, which is applied in the field of supramolecular chemistry, can solve the problems of difficult identification of metal ions, sensitivity to pH value of radius environment, etc., and achieves the effect of strong anti-interference performance and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 4
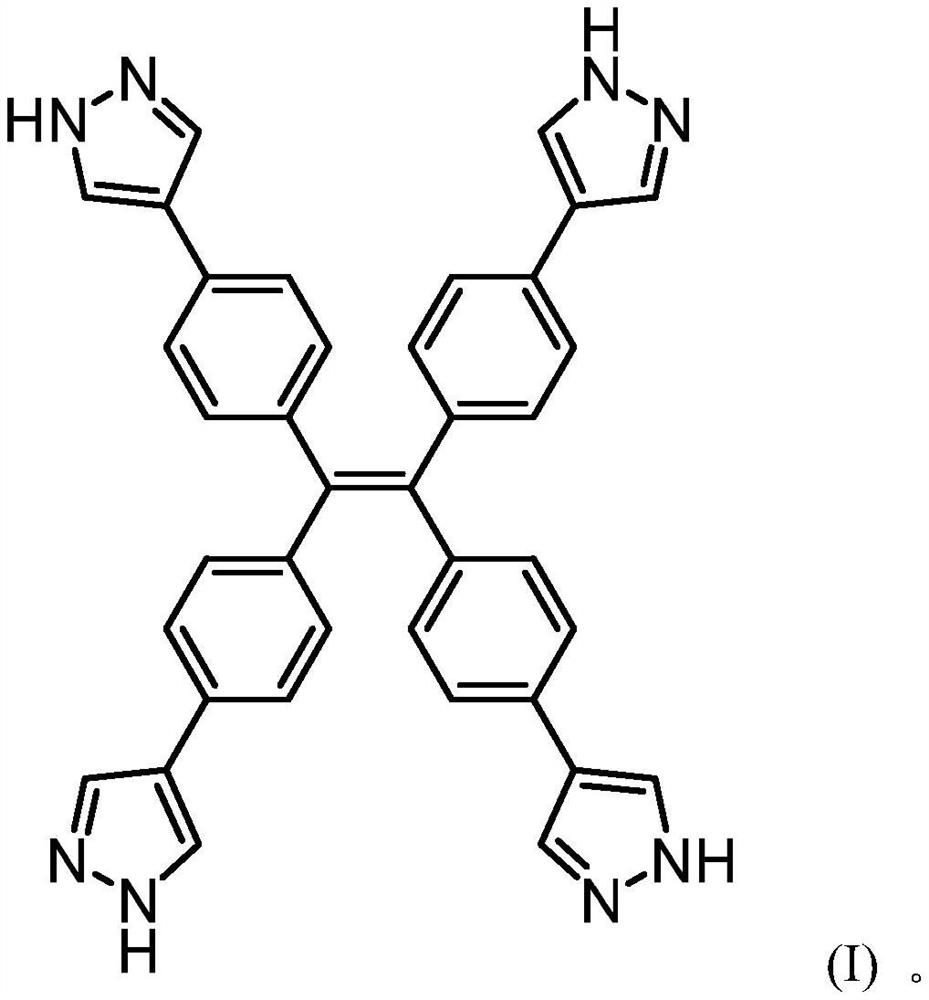
[0032] Synthesis of embodiment 1 tetrastyryl polypyrazole nitrogen-containing heterocyclic compound
[0033] Weigh 1,1,2,2-tetrakis(4-bromophenyl)ethylene (1.23g, 1.91mmol) and tetrakis(triphenylphosphine)palladium (330mg, 0.14mmol) in a 100mL three-necked flask, add 20mL n-Butanol and 10ml of water, install a reflux device, add 4mL of 2M potassium carbonate aqueous solution, add 1-(tetrahydropyran-4-yl)-1H-pyrazole-4-boronic acid under the protection of gas (nitrogen) The nicohol ester (4.00 g, 14.38 mmol) was stirred at 120° C. for 72 hours.
[0034] The solvent was distilled off under reduced pressure, the resulting product was dissolved in dichloromethane, extracted three times with water, the organic layer was taken, and the solvent was removed; the product 1,1,2,2-tetrakis(4-(1-(tetrahydro pyran-4-yl)-1H-pyrazol-4-yl)phenyl)ethylene. The product 1,1,2,2-tetrakis(4-(1-(tetrahydropyran-4-yl)-1H-pyrazol-4-yl)phenyl)ethene was dissolved in methanol 20ml, and dilute Hydroc...
Embodiment 2
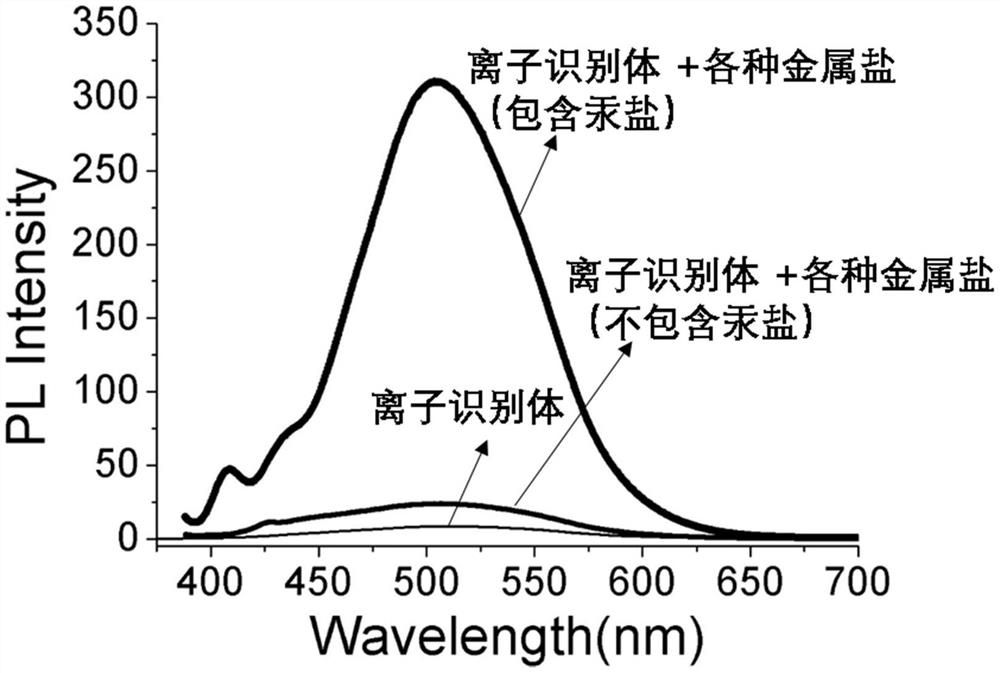
[0040] Embodiment 2 The selectivity of the ion recognition body of the present invention to metal mercury ions
[0041] Take 4.8 mg of the compound of formula (I) prepared by the present invention as an ion recognizer, add the ion recognizer into a sample bottle, and prepare a 0.01 mM clear solution with DMA. Prepare Hg(NO 3 ) 2 An aqueous solution of mercury ions, with a concentration of 0.01M, and a mixed aqueous solution of other transition metal ions, respectively Zn(NO 3 ) 2 , Cu(NO 3 ) 2 , Cd(NO 3 ) 2 , Co(NO 3 ) 2 , Ni(NO 3 ) 2 , Al(NO 3 ) 3 , Fe(NO 3 ) 3 , Ag(NO 3 ), Al(NO 3 ) 3 , K 2 CO 3 , the concentration is 0.01M.
[0042]Add this series of metal ion aqueous solutions to the system, only the addition of mercury ions causes the obvious solution color to turn green, and with 378nm as the fluorescence excitation wavelength, the fluorescence intensity at around 520nm is enhanced by about 15 times, see the attached figure 1 . This result shows tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com