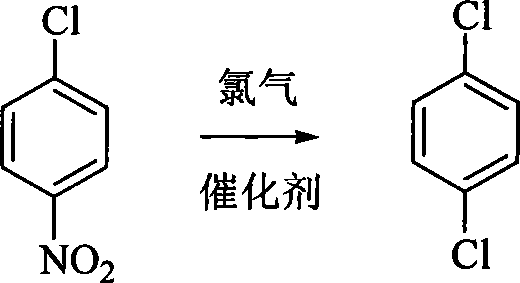
Method for synthesizing p-dichlorobenzene
A technology of p-dichlorobenzene and dichlorobenzene, which is applied in the field of synthesis of p-dichlorobenzene, can solve the problems of small scale, difficult separation, and poor product quality, and achieve the effects of mild reaction conditions, simple process operation, and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 15.8 grams (0.1 moles) of p-nitrochlorobenzene and 0.08 grams of catalyst benzoyl peroxide into the reaction flask, heat to 200 ° C, and feed chlorine gas to react for 6 hours to obtain the crude product of p-dichlorobenzene, which is rectified Column rectification, obtains the product 13.6 grams of purity ≥ 99.5%, yield 93%. The melting point is 52-53°C. The brown-red gas (nitroxyl chloride) produced during the reaction is absorbed with ammonia and ammonium chloride liquid.
Embodiment 2
[0024] Add 15.8 grams (0.1 moles) of p-nitrochlorobenzene and 0.08 grams of catalyst azobisisobutyronitrile into the reaction flask, heat to 200 ° C, and feed chlorine gas to react for 6 hours to obtain the crude product of p-dichlorobenzene. Distillation column rectification, obtains the product 13.8 grams of purity ≥ 99.5%, yield 94%. The melting point is 52-53°C. The brown-red gas (nitroxyl chloride) produced during the reaction is absorbed with ammonia and ammonium chloride liquid.
Embodiment 3
[0026] Add 15.8 g (0.1 mole) of p-nitrochlorobenzene and 0.1 g of catalyst azobisisobutyronitrile into the reaction flask, heat to 150° C., and feed chlorine gas to react for 6 hours to obtain the crude p-dichlorobenzene, which is refined and purified. Distillation column rectification, obtains the product 13 grams of purity ≥ 99.5%, yield 89%. The melting point is 52-53°C. The brown-red gas (nitroxyl chloride) produced during the reaction is absorbed with ammonia and ammonium chloride liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com