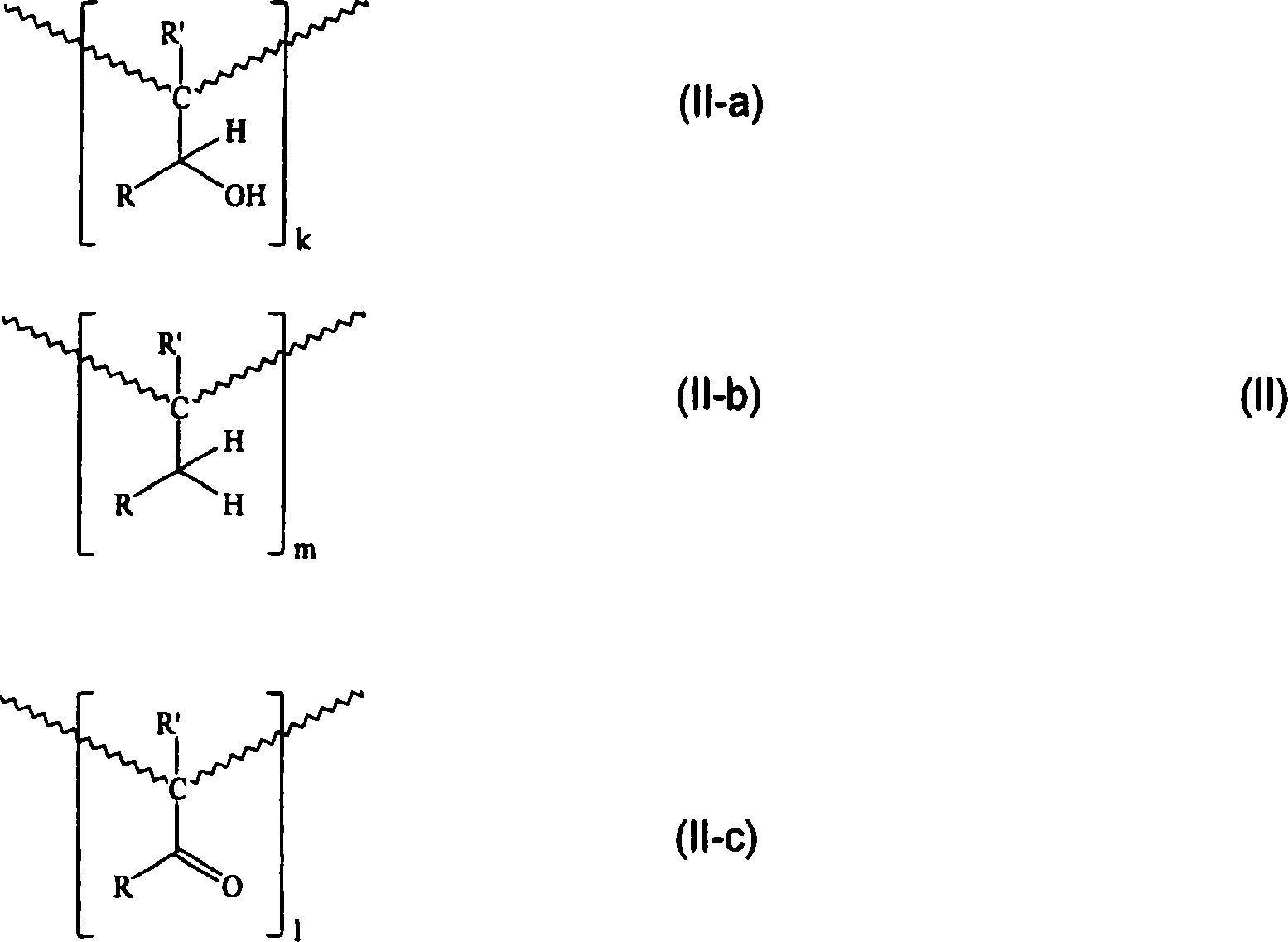
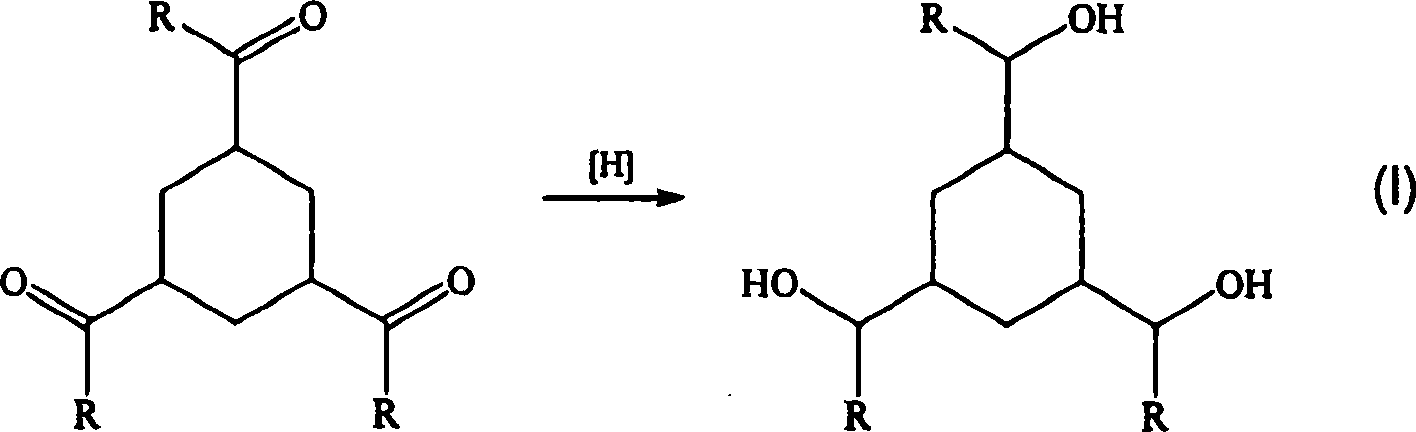
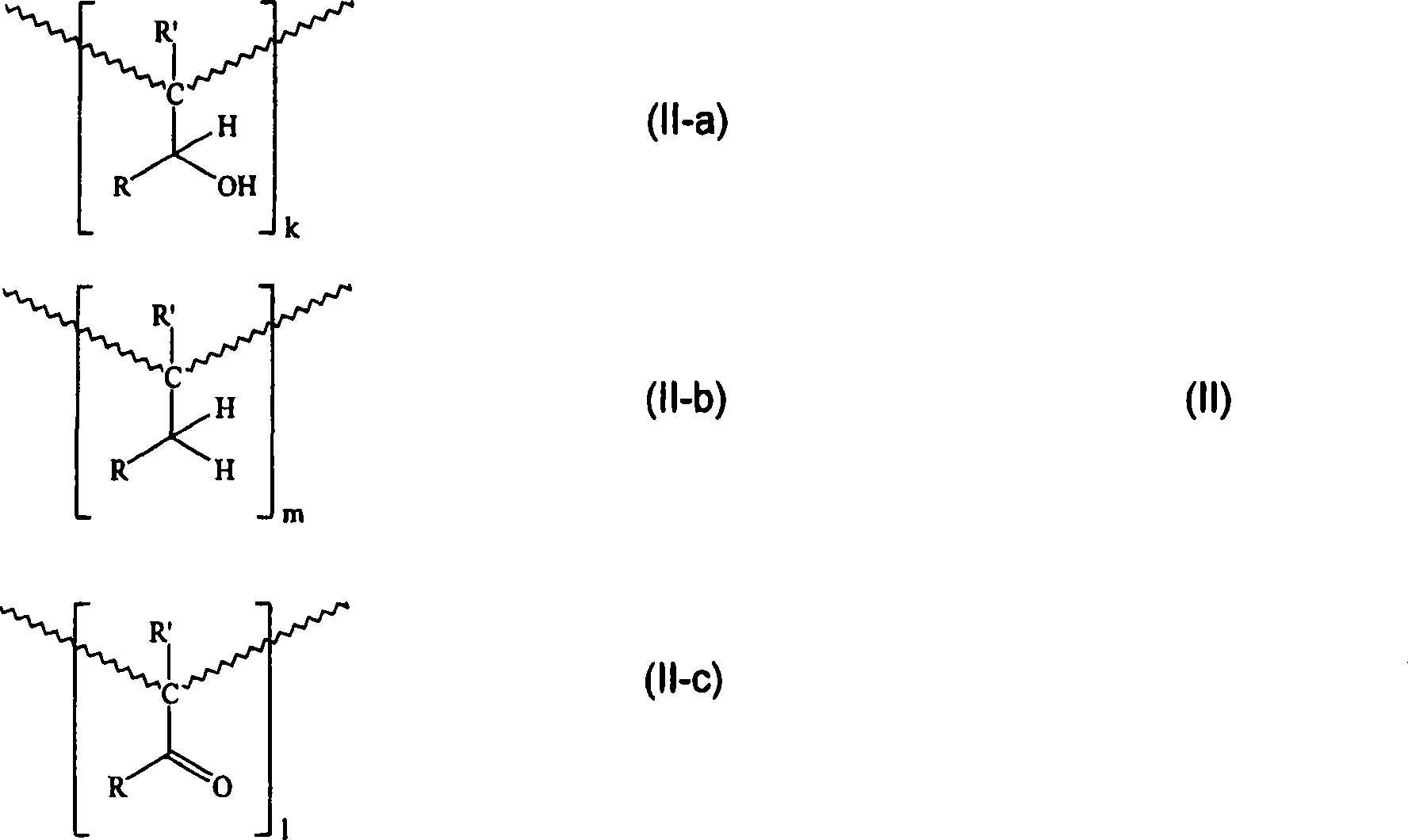Formaldehyde-free, oh-functional, carbonyl- and ring-hydrogenated ketone-aldehyde resins based on alkyl aryl ketones and formaldehyde and a process for preparing them
An alkyl aryl ketone, hydroxyl functional technology, applied in the field of ketone-aldehyde resin and its preparation, can solve the problem of low fraction of crystallizable compounds, and achieve the effects of excellent light stability, thermal stability and low color.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0173] Non-invention, comparative example
[0174] The document which best describes the prior art is DE 33 34 631 A1.
[0175] The acetophenone / formaldehyde resin used here was obtained according to Example 2 of DE 892 975 .
Embodiment A
[0176] Example A: Repetition of Example 2 of DE 892 975
[0177] 1200 g of acetophenone were mixed with 240 g of a 50 wt % strength aqueous potassium hydroxide solution and 400 g of methanol, and subsequently, over a period of 2 h with vigorous stirring, 1000 g of a 30 wt % strength formaldehyde solution. During this addition, the temperature increased to 90°C. This temperature was maintained for 10 h. The batch was acidified with sulfuric acid, and the resulting condensation product was washed with hot water, melted, and dried under reduced pressure.
[0178] 1260 g of yellow resin were thus obtained. The resin is transparent and brittle with a melting point of 67°C. The Gabon color value is 3.8 (50% by weight in ethyl acetate). It is soluble, for example, in acetates such as butyl acetate and ethyl acetate, and in aromatic hydrocarbons such as toluene and xylene. It is insoluble in ethanol. The formaldehyde content is 255ppm.
Embodiment B
[0179] Example B: Repetition of Example 3 of DE 33 34 631 A1
[0180]The resin from Example A was dissolved in isobutanol by heating at a concentration of 30% by weight. The hydrogenation reaction was carried out in a continuously operating fixed-bed reactor filled with 400 mL of an industrially used nickel catalyst (Engelhard Ni 5126T1 / 8). This catalyst, according to Engelhard, is identical to the catalyst "Harshaw Ni5124" used in DE 33 34 631 . At 300 bar and 180° C., 250 mL / h of the reaction mixture was sent through the reactor from top to bottom (trickle mode). The pressure is maintained constant by a supplemental supply of hydrogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com