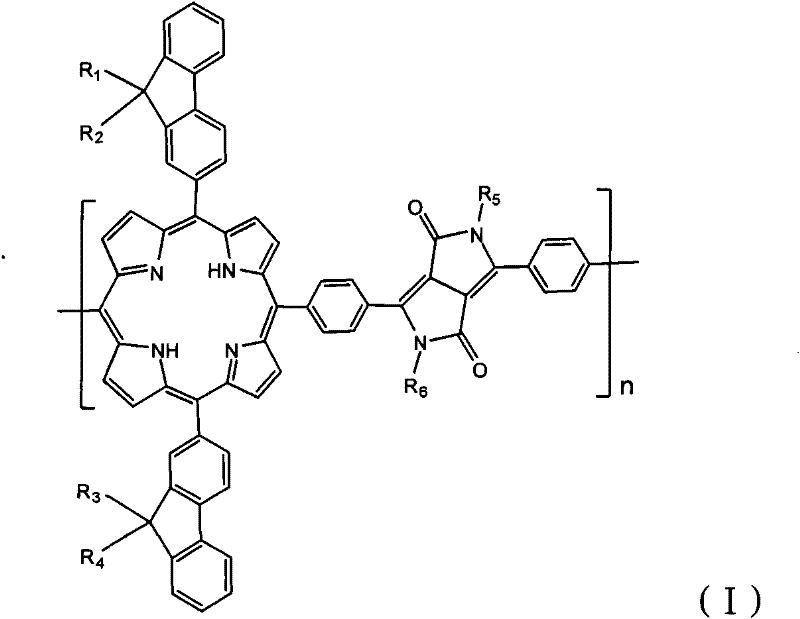
Porphyrin copolymer containing diketopyrrolopyrrole unit, preparation method and application thereof
A technology of diketopyrrolopyrrole and copolymer, which is applied in the field of preparation of porphyrin copolymer containing diketopyrrolopyrrole units, can solve the problems of narrow material spectral response, low hole mobility, poor stability, etc., and achieve High quantum efficiency, reduced process flow, and increased density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
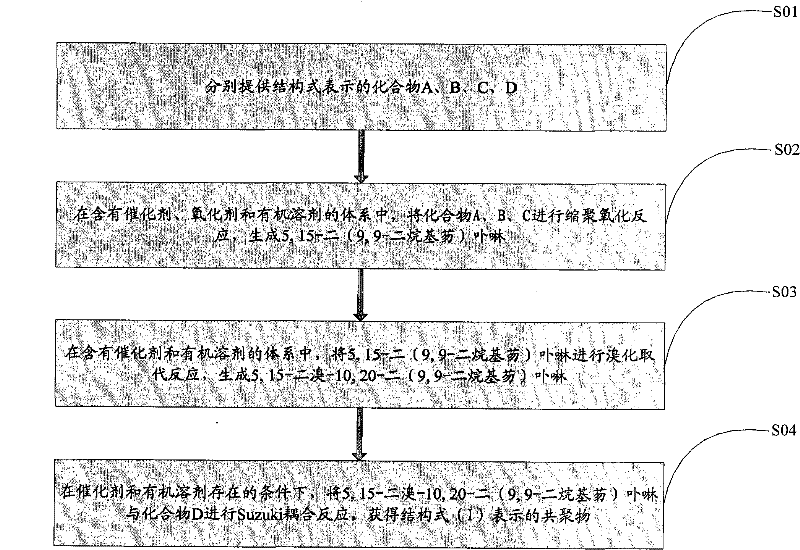
[0033] see figure 2 , the preparation method of the above-mentioned porphyrin copolymer containing diketopyrrolopyrrole units comprises the following steps:
[0034] S01: respectively provide compounds A, B, C, and D represented by the following structural formulas,
[0035]
[0036] Among them, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 from C 1 -C 32 the alkyl group;
[0037] S02: In an organic solvent system containing a catalyst and an oxidizing agent, the compounds A, B, and C are subjected to a polycondensation oxidation reaction to generate 5,15-bis(9,9-dialkylfluorene)porphyrin;
[0038] S03: In an organic solvent system containing a catalyst, 5,15-bis(9,9-dialkylfluorene)porphyrin is subjected to a bromination substitution reaction to generate 5,15-dibromo-10,20-bis(9 , 9-dialkylfluorene) porphyrin;
[0039] S04: Under an inert gas environment and in an organic solvent system containing a catalyst, 5,15-dibromo-10,20-bis(9,9-dialkylfluorene)porphyrin and compoun...
Embodiment 1
[0087] In the copolymer (I-1) of this embodiment, R 1 , R 2 , R 3 , R 4 , R 5 , R 6 Same as C 8 h 17 , its structural formula is as follows:
[0088]
[0089] It can be seen from the structural formula that the porphyrin copolymer containing diketopyrrolopyrrole units in the first embodiment has a symmetrical bifluorenyl structure. Through this uniform and symmetrical structure, the porphyrin copolymer containing diketopyrrolopyrrole units can be copolymerized The material has better light-absorbing properties and photoelectric properties. due to R 1 , R 2 , R 3 , R 4 Both use C 8 h 17 , so compounds A and B are both 9,9-dioctyl-2-aldehyde fluorene, so only one kind of raw material is needed, which simplifies the preparation process and reduces the cost, and the yield is high. If it is not such a symmetrical structure, then compounds A and B have different structures, different raw materials need to be obtained, and there are relatively more by-products.
[009...
Embodiment 2
[0113] In the copolymer (I-2) of the present embodiment, R 1 for CH 3 , R 2 for C 8 h 17 , R 3 for C 10 h 21 , R 4 for C 16 h 33 , R 5 for C 8 h 17 , R 6 for C 16 h 33 , its structural formula is as follows:
[0114]
[0115] The preparation process is described in detail below.
[0116] Step 1, the preparation of 10-(9-methyl-9-octylfluorene)-20-(9-decyl-9-hexadecylfluorene) porphyrin, the reaction formula is as follows:
[0117]
[0118] The specific process is as follows: Prepare an anhydrous and oxygen-free device, weigh 0.320g 9-methyl-9-octylfluorene, 0.56g 9-decyl-9-hexadecylfluorene, 0.30g di Pyrromethane was dissolved in dichloromethane, nitrogen gas was introduced for 30 minutes, 2 mL of trifluoroacetic acid was added with a syringe, stirred at room temperature for 3 hours, then 1.82 g was added into DDQ, stirred at room temperature for 30 minutes, and finally 2 mL of triethylamine was added to quench After the reaction, the solvent was concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com