Method for taking off endotoxin in primary pure plasmids or proteins, and kit
A protein and endotoxin technology, applied in the field of biomedicine, can solve the problems of unreachable, affected yield, and poor removal of plasmid endotoxin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
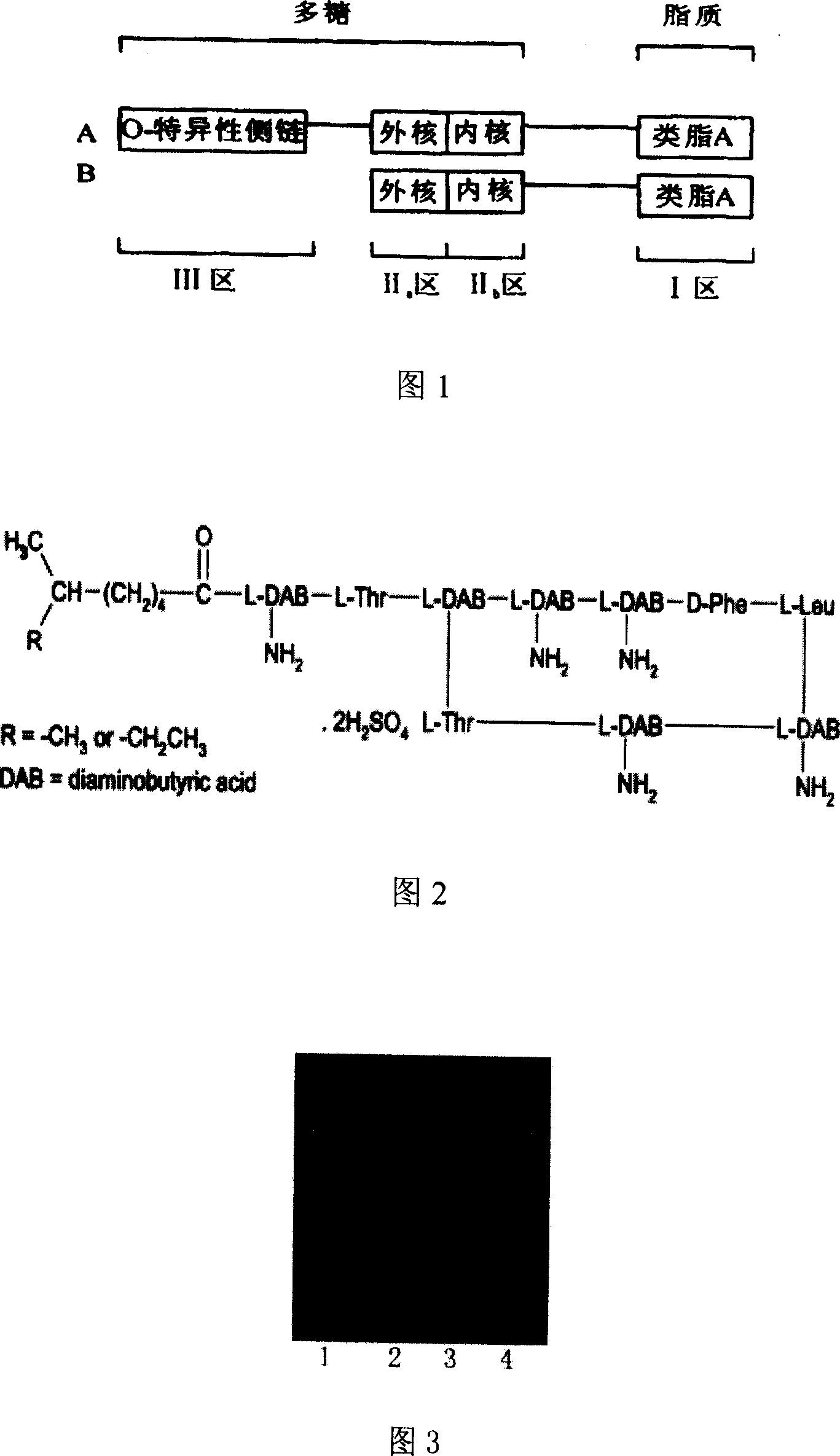
[0041] The content of the present invention will be described in more detail below in conjunction with the accompanying drawings and specific embodiments, and the present invention will be further elaborated, but these embodiments are by no means limiting the present invention. Any changes made by those skilled in the art in the implementation of the present invention under the inspiration of this specification will fall within the scope of the claims of the present invention.
[0042] Experimental Materials and Instruments
[0043] Primary purified plasmids: HG43, PSW, PSA43 and Ag85A recombinant protein solutions were obtained from Shanghai Haigui Biotechnology Co., Ltd.
[0044] Organic solvents: isopropyl myristate, ethyl acetate, diethylene glycol monomethyl ether, benzyl benzoate, chloroform, Analytical grades were purchased from China Pharmaceutical Group Shanghai Chemical Reagent Company. Polymyxin B sulfate (PMXB) and Triton-X-114 were purchased from Sigma (P1004 a...
Embodiment 1
[0048] Example 1 Determination of endotoxin content in plasmid liquid by semi-quantitative method for gelation of limulus reagent
[0049] 1. Recheck the sensitivity of LAL reagent : Adopt the Limulus test kit (sensitivity is 0.25EU / ml) and endotoxin standard substance (10EU / ml) produced by Zhanjiang Andus Company, operate according to the manufacturer's instructions, and the check results are shown in Table 1.
[0050] Table 1 Limulus Reagent Sensitivity Review
[0051] endotoxin
Standard solution concentration
4λ
1EU / ml
2λ
0.5EU / ml
0.25EU / ml
0.5λ
0.125EU / ml
Negative
sex
gelled result
++++
++++
++++
----
--
[0052] The above experimental results show that the sensitivity of the Limulus reagent used is consistent with the marked value, and it is used according to the marked value.
[0053] 2. Serial dilution of plasmid sample...
Embodiment 2
[0061] Example 2 Triton Fog Point Centrifugal Phase Separation Method for Removing Endotoxin in Plasmid Liquid
[0062] This example is to repeat the steps described in the literature (Cotten, Baker A, Sallik M, et al.: GeneTherapy, 1: 239-46, 1994), in order to verify the effect of this method on removing endotoxin.
[0063] Take 1ml of the initial pure HG43 plasmid solution with a concentration of 1.2mg / ml (prepared with 0.1M PBS buffer solution) and add it to a clean centrifuge tube. Add TritonX-114 (undiluted). Mix at 4-10°C for 15 minutes, then move to 35-37°C and let it stand for 5 minutes. After the mixture becomes cloudy and turbid, centrifuge at 13,000rpm in a desktop centrifuge at the same temperature for 5 minutes, and oily liquid deposits at the bottom of the small tube (for TritonX -114 complex with endotoxin). Carefully draw the upper aqueous phase containing the plasmid (no oily liquid), transfer it to another clean vial, add 20μl TritonX-114, mix at 4-10°C fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com