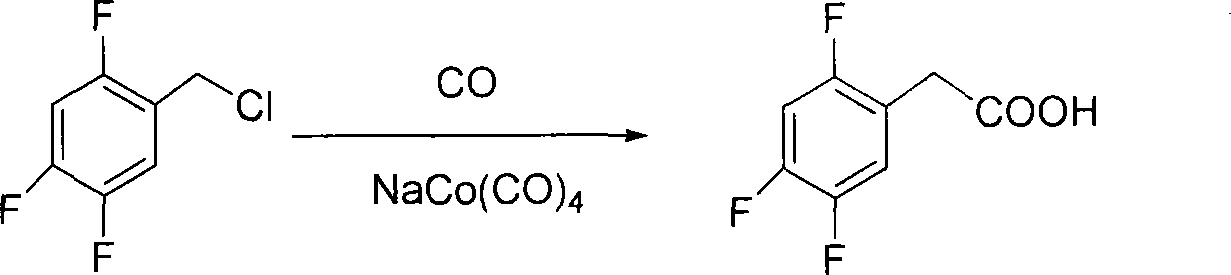Method for preparing 2,4,5 trifluorobenzene acetic acid
A technology of trifluorophenylacetic acid and trifluorobenzene, which is applied in two fields, can solve the problems of long steps, unstable Grignard reagents, and large pollution, and achieve the effects of mild reaction conditions, low reagent prices, and avoiding potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
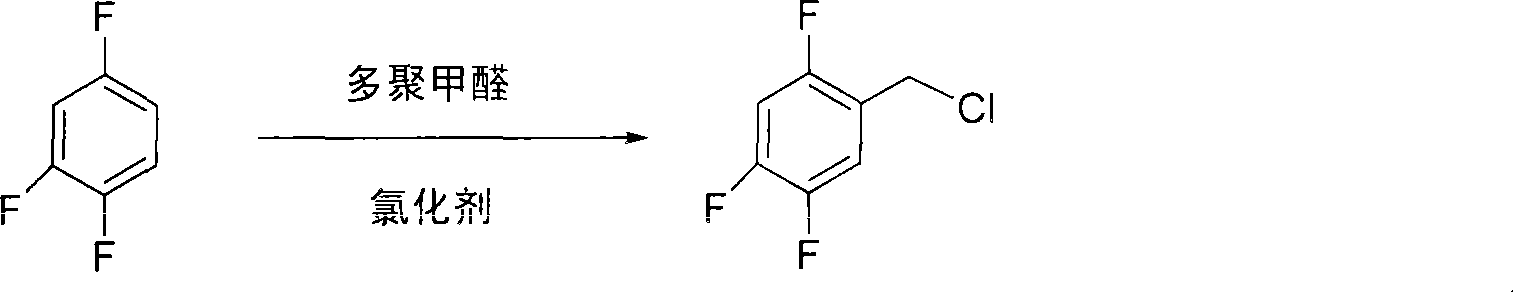
[0011] (1) Preparation of 2,4,5-trifluorobenzyl chloride
[0012] Add 120 mL of hydrochloric acid, 70 g of zinc chloride and 31.2 g of paraformaldehyde (1.04 mol) into a three-necked flask, add 106 g (0.8 mol) of 1,2,4-trifluorobenzene with stirring at room temperature, and keep the reaction for 5 hours. Separate the organic phase, wash with water until it is neutral, and distill under reduced pressure to obtain 98.5 g of 2,4,5-trifluorobenzyl chloride with a content of 99% and a yield of 68%.
[0013] The reaction equation is:
[0014]
[0015] (2) Preparation of 2,4,5-trifluorophenylacetic acid
[0016] Add 35mL of the methanol solution of sodium cobalt tetracarbonyl (8.8mmol) into the reactor that has already contained 150mL of methanol, seal the reactor and fill with CO gas, and pump 100 grams (0.55mol) when the temperature reaches 40℃. , 5-trifluorobenzyl chloride and 120 grams of 40% sodium hydroxide solution, the reaction is maintained for 2 hours. Add 300 mL of water, di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com