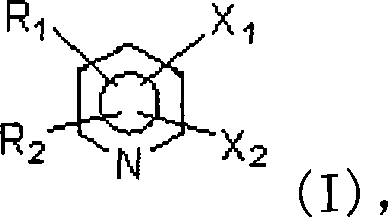
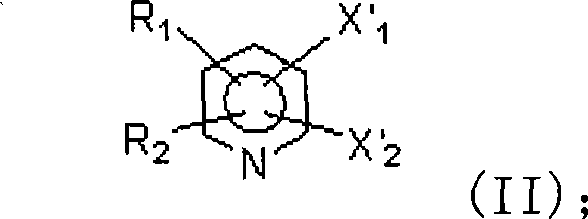
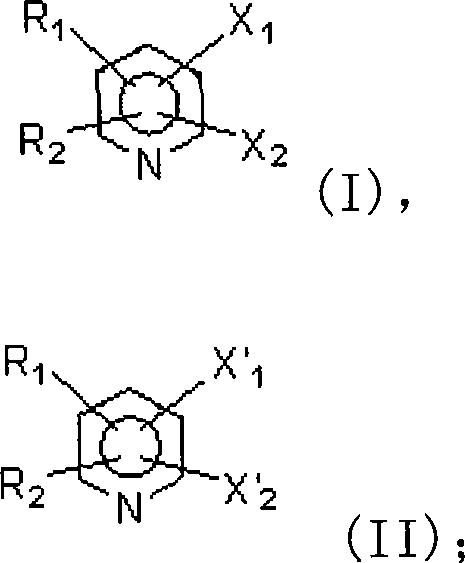
Method for preparing a category of compound of trifluoro methylpyridine
A technology of trifluoromethylpyridine and trichloromethylpyridine is applied in the field of preparation of trifluoromethylpyridine compounds, and can solve the problems of difficulty in obtaining, high reaction temperature, and difficulty in industrial realization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0099] (2) The preparation method of the present invention reacts with raw materials and catalysts that are easily available in industry with low price, stable chemical properties, and difficult to decompose by moisture absorption. The raw and auxiliary materials of the method are cheap and easy to obtain, the process is simple, and the aftertreatment is easy. The condition is mild, the environmental pollution is small, the trifluoromethylpyridine compound can be prepared with a relatively high yield, and industrialization is easy at the same time.
[0100](3) Catalysts in the prior art: high-valent metal chlorides are very easy to decompose by hygroscopicity, are very difficult to prepare, and are very expensive. At present, there are no commercial products in China. However, the present invention adopts a new catalyst for chlorination reaction, thereby achieving higher yield, lowering cost and reducing environmental pollution.
Embodiment 1
[0104] Put into 600g (2.2mol) 2,3-dichloro-5-trichloromethylpyridine (molecular weight 265.35g / mol), 18g (3%wt)MoO 3 , stir well, at T 内 =150 DEG C, pass into HF in the above-mentioned solution, stop passing through HF after 10 hours, GC tracks and detects reaction mixture (weight %):
[0105] 3-Chloro-2-fluoro-5-trifluoromethylpyridine: 12.9%
[0106] 2,3-Dichloro-5-trichloromethylpyridine: 34.6%
[0107] 3-chloro-2-fluoro-5-chlorodifluoromethylpyridine: 8.6%
[0108] 2,3-Dichloro-5-chlorodifluoromethylpyridine: 43.4%
[0109] Add 100g raw material 2,3-dichloro-5-trichloromethylpyridine, at T 内 =150°C, continue to stir and keep warm for 4 hours to complete the reaction. GC detection reaction mixture (weight %):
[0110] 3-Chloro-2-fluoro-5-trifluoromethylpyridine: 0.46%
[0111] 2,3-Dichloro-5-trifluoromethylpyridine: 72.8%
[0112] 3-chloro-2-fluoro-5-chlorodifluoromethylpyridine: 12.4%
[0113] 2,3-Dichloro-5-dichlorofluoromethylpyridine: 4.5%
[0114] 2,3-Dichlor...
Embodiment 2
[0117] Drop into 660g (2.5mol) 2,3-dichloro-5-trichloromethylpyridine (molecular weight 265.35g / mol), 19.8g (3%wt) MoO in 1 L four-necked bottle 2 , stir well, at T 内 =170 DEG C, pass into HF in above-mentioned solution, stop passing through HF after 20 hours, GC tracks and detects reaction mixture (weight %):
[0118] 3-Chloro-2-fluoro-5-trifluoromethylpyridine: 1.1%
[0119] 2,3-Dichloro-5-trifluoromethylpyridine: 66.3%
[0120] 3-chloro-2-fluoro-5-chlorodifluoromethylpyridine: 16.2%
[0121] 2,3-Dichloro-5-dichlorofluoromethylpyridine: 8.7%
[0122] 2,3-Dichloro-5-trichloromethylpyridine: 7.6%
[0123] After the reaction, the crude product of 2,3-dichloro-5-trifluoromethylpyridine was adjusted to PH=8-9, washed, separated, dried, and distilled to obtain 412.7g of the product with a content of 99.6% and a yield of 73.8%. The boiling point b.p. is 80°C (20mmHg).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com