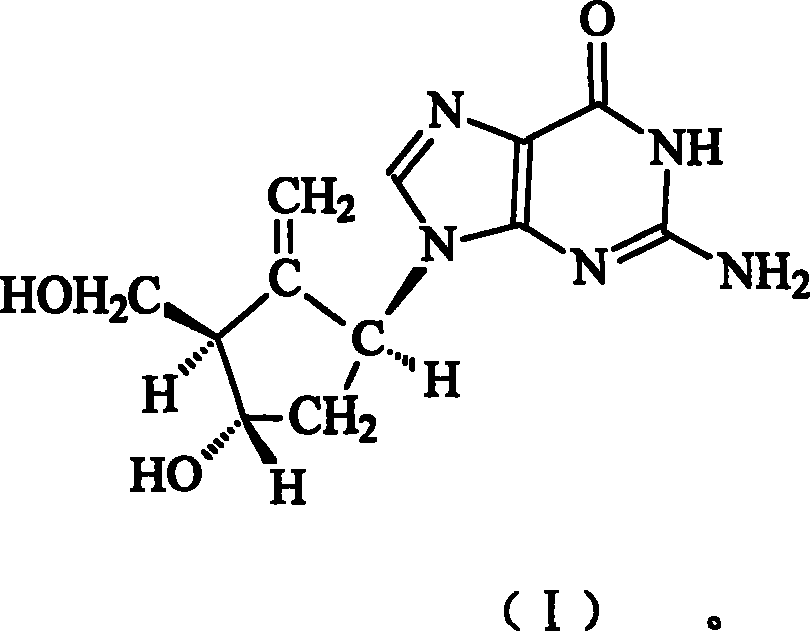
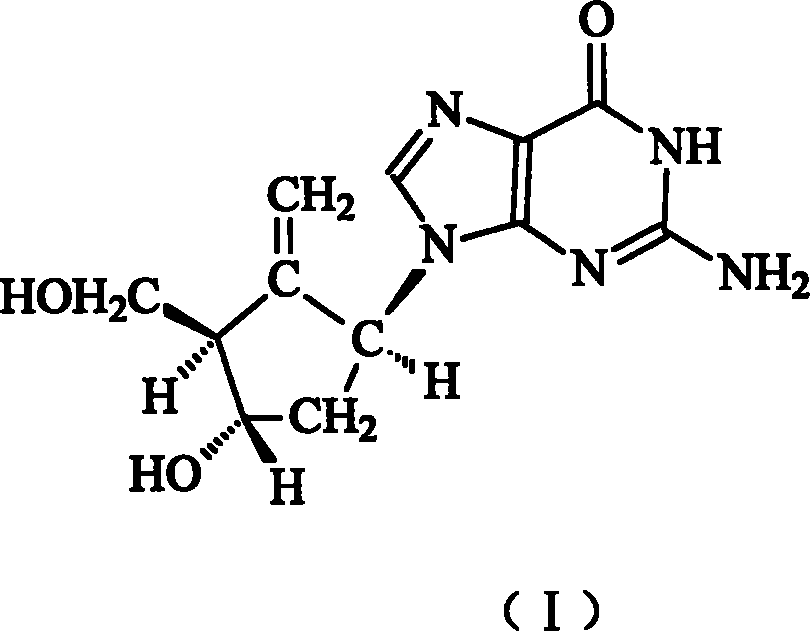
Entecavir medicinal composition and uses thereof
A technology of eltecavir and composition, which is applied in the field of eltecavir pharmaceutical composition, can solve the problems of viral rebound, the emergence of patients, and the comparison of short-term effects of eltecavir, etc., and achieves far-reaching social benefits and broad application. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The composition of embodiment 1 etecavir silymarin and its derivatives
[0024] Etecavir is mixed with silymarin and its derivatives (extracted from Compositae Silybum marianum L. Gaertn) in a certain ratio to prepare a composition.
[0025] The duck hepatitis B model test was carried out, and the results showed that it had an obvious effect of inhibiting DHBV, and the DHBV DNA was inhibited by 80% on the 10th day of administration, and the edema of the liver pathology section was significantly reduced;
[0026] The results of various animal experimental liver injury tests show that the above pharmaceutical composition has a protective effect on experimental liver injury, can resist necrosis of liver cells, reduce fatty degeneration, and restore the damage of mitochondria and endoplasmic reticulum in liver cells.
Embodiment 2
[0027] Embodiment 2, the composition of etecavir glycyrrhizic acid and its derivatives
[0028] Etecavir and glycyrrhizic acid are extracted from the dry rhizome of leguminous plant Glycyrrhiza uralensis Fisch or Glycyrr-hiza Glabra) in a certain ratio to obtain the composition, and the duck hepatitis B model test is carried out. The results show that animal experiments show that its anti- Hepatitis B virus is strong. The composition has a protective effect on the rat liver injury model of experimental animals, can reduce ALT, alleviate histological lesions, and can obviously improve the deep jaundice of experimental animals.
Embodiment 3
[0029] Embodiment 3, etecavir matrine and etecavir oxymatrine composition
[0030] Etecavir is mixed with matrine and oxymatrine (extracted from Sophorae flavescentis Ait, Sophora alopecuroids L and Sophorae tonkinensis Gapnep) in a certain ratio to prepare a composition. According to the in vitro HepG2 2.215 cell test, it can inhibit HBsAg by 80.5% and HBeAg by 68.0% after 10 days of administration; the duck hepatitis B model test shows that it has obvious inhibitory effect on DHBV-DNA, and the DHBV DNA is inhibited by more than 70% on the 10th day of administration After liver cell injury and liver fibrosis caused by carbon tetrachloride, galactosamine, ethanol, etc. in rats, 90% of serum bilirubin returned to normal, and 80% of ALT decreased, especially for jaundice.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com