Process for production of aromatic compound and process for production of hydrogenated aromatic compound
A technology for aromatic compounds and production methods, applied in organic chemical methods, hydrocarbon production from carbon oxides, condensation hydrocarbon production with dehydrocarbons, etc., can solve problems such as inability to obtain reaction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
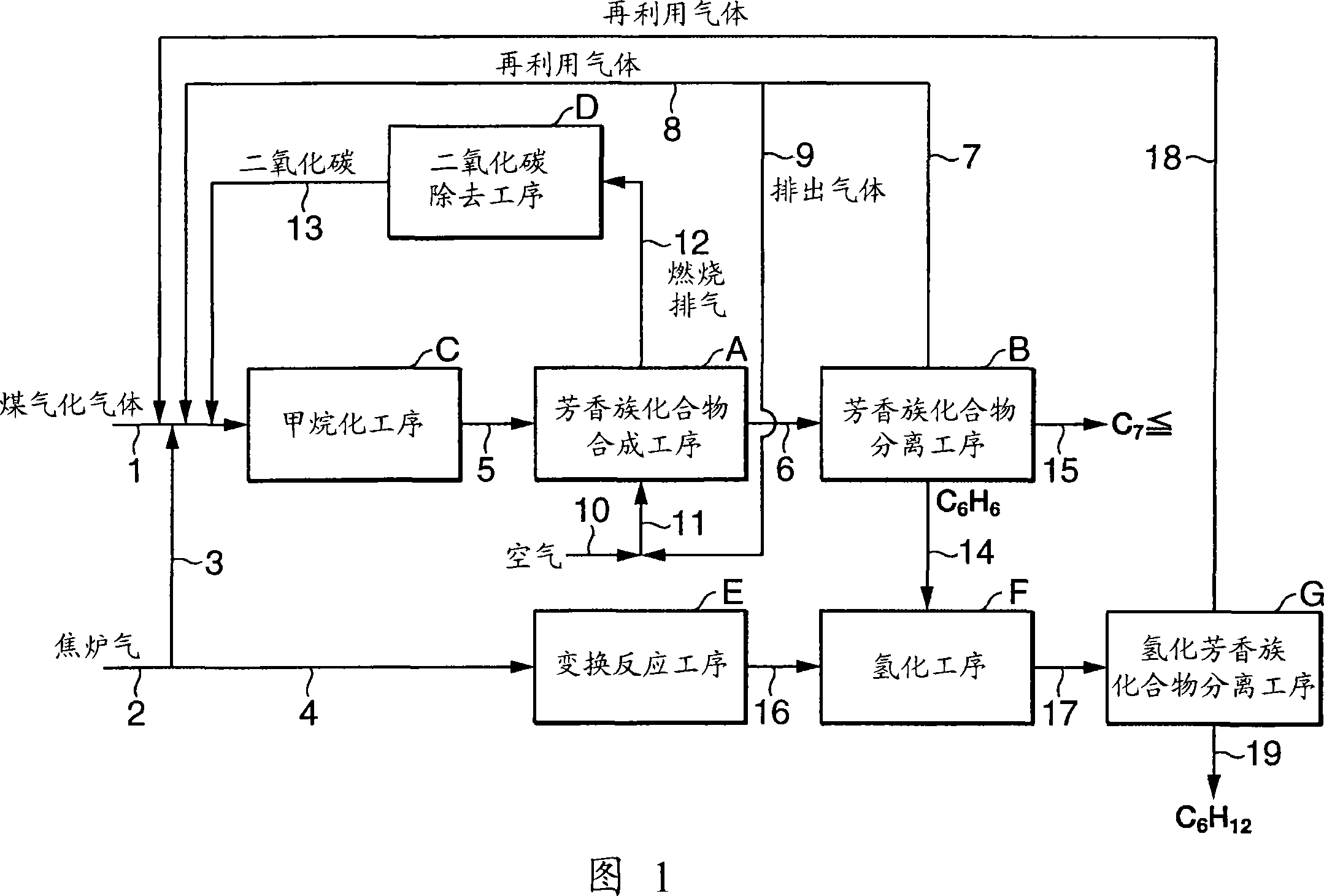
[0133] Using coal gasification gas (H 2 , CO, CO 2 , N 2 ) and coke oven gas (H 2 、CH 4 , CO, CO 2 , N 2 ) as the raw material gas supplied from the outside, according to the flow chart shown in Figure 1, aromatic compounds are produced continuously. The coke oven gas is used after pretreatment of desulfurization, detarring and dedusting according to the usual method.
[0134] Furthermore, the aromatic compound synthesis catalyst was prepared according to the method in Example 2 of Japanese Patent Laid-Open No. 2005-255605. That is, as the catalyst raw material, use inorganic component: organic binder: water = 65.4: 13.6: 21.0 (weight ratio), and the inorganic component is SiO 2 / Al 2 o 3(Molar ratio)=40 ZSM-5 zeolite (MFI type zeolite):clay:glass fiber=82.5:10.5:7.0 (weight ratio) mixture. First, the catalyst raw material was kneaded to form a molded body, and then, after drying at 100°C for 5 hours, it was calcined at 750°C. Next, the obtained sintered body was im...
Embodiment 2
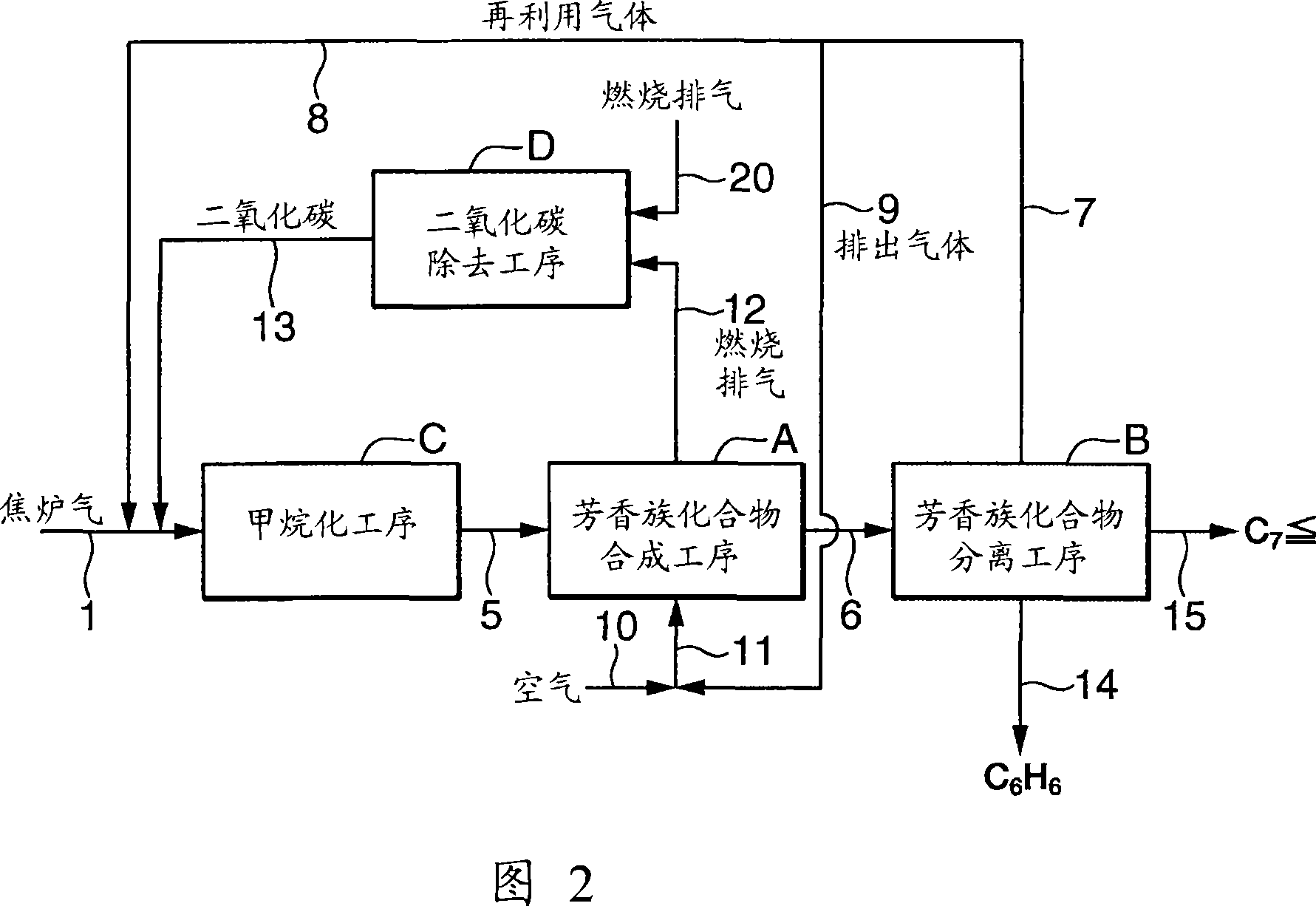
[0161] Using coke oven gas (H 2 、CH 4 , CO, CO 2 , N 2 ) as a raw material gas supplied from the outside, according to the flowchart shown in FIG. 2 , aromatic compounds are continuously produced. The coke oven gas is used after pretreatment of desulfurization, detarring and dedusting according to the usual method.
[0162]
[0163] In the methanation step (C), the coke oven gas is supplied from the line (1), and the recycled gas (gas containing methane and hydrogen) from the aromatic compound separation step (B) described later is supplied from the line (8). , Carbon dioxide is supplied from line (13). The condition of methanation process (C) is pressure: 10kg / cm 2 , Temperature (inlet): 280°C, GHSV: 300h -1 . Table 6 shows the composition of the gas supplied to the methanation step and the composition of the gas (methane-containing gas) produced in the methanation step (composition after cooling to 40° C. with a cooler and separating condensed water).
[0164] Tabl...
Embodiment 3
[0178] Using coke oven gas (H 2 、CH 4 , CO, CO 2 , N 2) and carbon dioxide recovered from the combustion exhaust gas as the raw material gas supplied from the outside, according to the flow chart shown in Figure 2, aromatic compounds are produced continuously. The coke oven gas is used after pretreatment of desulfurization, detarring and dedusting according to the usual method.
[0179]
[0180] In the methanation step (C), the coke oven gas is supplied from the line (3), and the recycled gas (lower hydrocarbons and hydrogen-containing gas) from the aromatic compound separation step (B) described later is supplied from the line (8). ), carbon dioxide is supplied from the line (13), and recycled gas (lower hydrocarbons and hydrogen-containing gas) from the hydrogenated aromatic compound separation step (G) described later is supplied from the line (18). These gases are all supplied to the methanation process (C) via the line (1). The condition of methanation process (C) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com