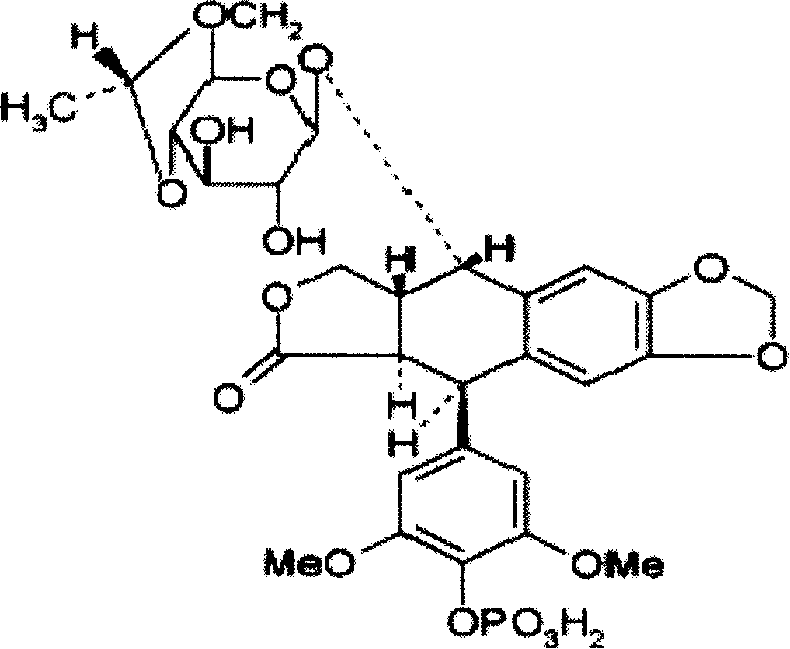
Etoposide venous emulsion and preparation method thereof
A technology of etoposide and emulsion, which is applied in the field of intravenous emulsion, and can solve the problems of poor water solubility, low bioavailability and low solubility of oral preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] First, dissolve 0.8 etoposide in methyl benzoate, heat in a constant temperature water bath at 80 degrees Celsius, add 12 g of soy lecithin, stir thoroughly for 3 minutes to disperse the soy lecithin evenly, add 100 g of soybean oil preheated to 80 degrees to the above liquid, and use The colostrum is produced by a high-speed emulsifier, and the preheated water for injection is added to the colostrum to a sufficient amount, and the volume is adjusted to 1000ml, transferred to a homogenizer for homogenization, and then filtered, potted and sterilized.
Embodiment 2
[0020] First, dissolve 1.0 etoposide in methyl benzoate, heat in a constant temperature water bath at 80 degrees Celsius, add 12 g of egg yolk lecithin, and stir for 3 minutes to make the soybean lecithin evenly dispersed, add 100 g of soybean oil preheated to 80 degrees Celsius to the above liquid, Use a high-speed emulsifier to make colostrum, add preheated water for injection to the colostrum to a sufficient amount, set the volume to 1000ml, transfer to a homogenizer for homogenization, and then filter, pot and sterilize.
Embodiment 3
[0022] First, dissolve 1.2 etoposide in methyl benzoate, heat in a constant temperature water bath at 80 degrees Celsius, add 12 g of soy lecithin, and stir for 3 minutes to make the soy lecithin evenly dispersed, add 100 g of soybean oil preheated to 80 degrees Celsius to the above liquid, and use The colostrum is produced by a high-speed emulsifier, and the preheated water for injection is added to the colostrum to a sufficient amount, and the volume is adjusted to 1000ml, transferred to a homogenizer for homogenization, and then filtered, potted and sterilized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com