Production of alkylated aromatic hydrocarbons from methane
A technology of alkylation and alkylating agent, which is applied in the production of liquid hydrocarbon mixtures, condensation hydrocarbons with dehydrocarbons, organic chemistry, etc., and can solve problems such as uneconomic recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
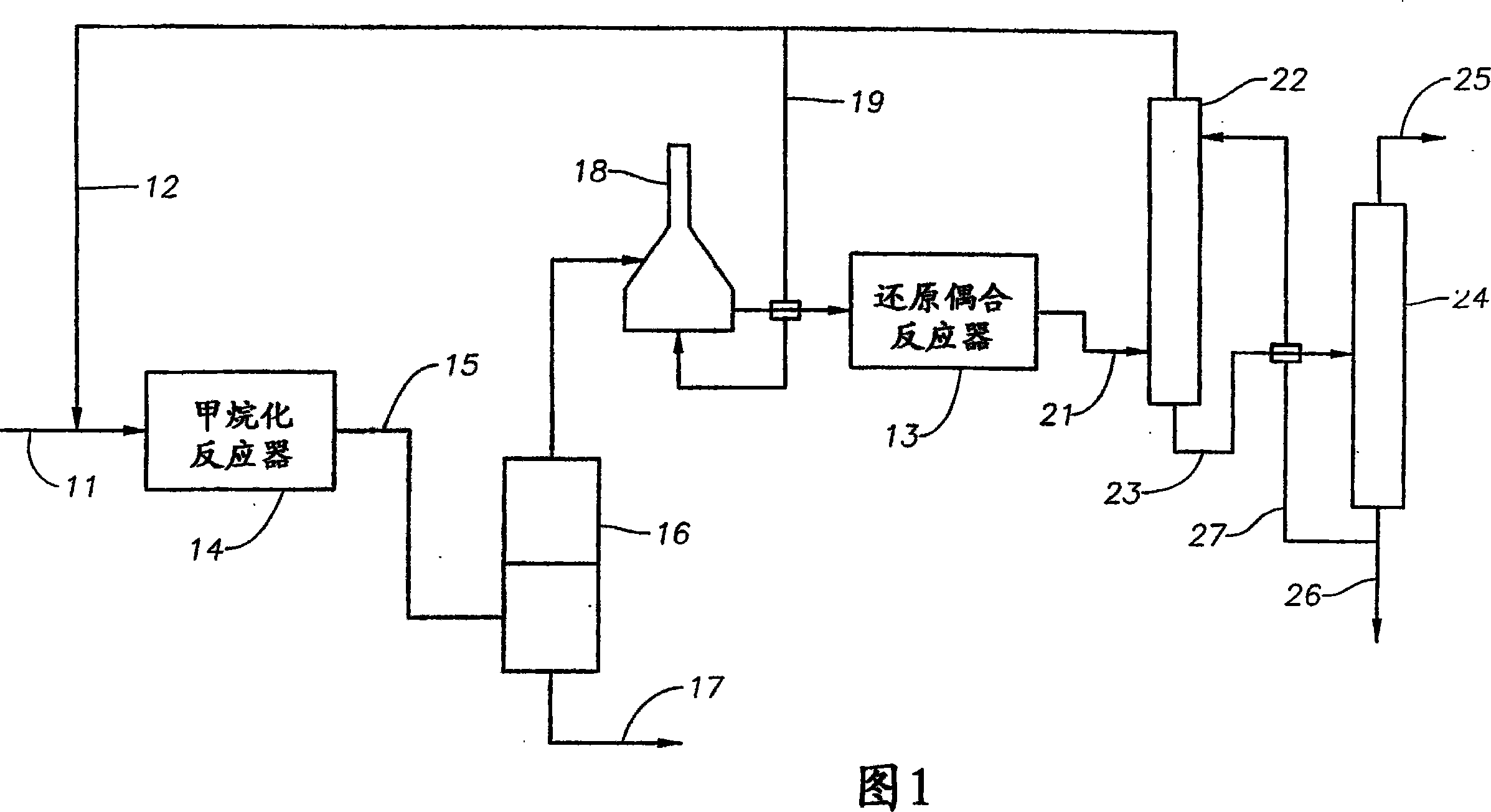
[0177] In one embodiment of the first example of the invention shown in Figure 1, the feed 11 is a natural gas stream containing 30.5 wt% carbon dioxide. Reactor 14 contained a Cu / Zn catalyst and was operated at a temperature of about 300°C and a pressure of 300 psia (2070 kPa). Reductive coupling reactor 13 contained MoZSM-5 catalyst and was operated at a temperature of about 900°C and a pressure of 50 kPa. The aromatic product separated by fractionation column 24 contained 90 wt% benzene and 10 wt% naphthalene-containing heavy fraction.
[0178] 100kg of benzene and 92kg of synthesis gas (by 81kg CO, 11kg H 2 composition) are combined and fed to an alkylation reactor containing supported Cr mixed with a silicon-selective PZSM-5 catalyst 2 o 3 / ZnO catalyst, and operate at a temperature of about 500°C and a pressure of 400 psia (2760 kPa). Separation of the alkylation effluent to remove C 8 The product stream will then be depleted in C 8 The effluent is divided into ben...
Embodiment 2
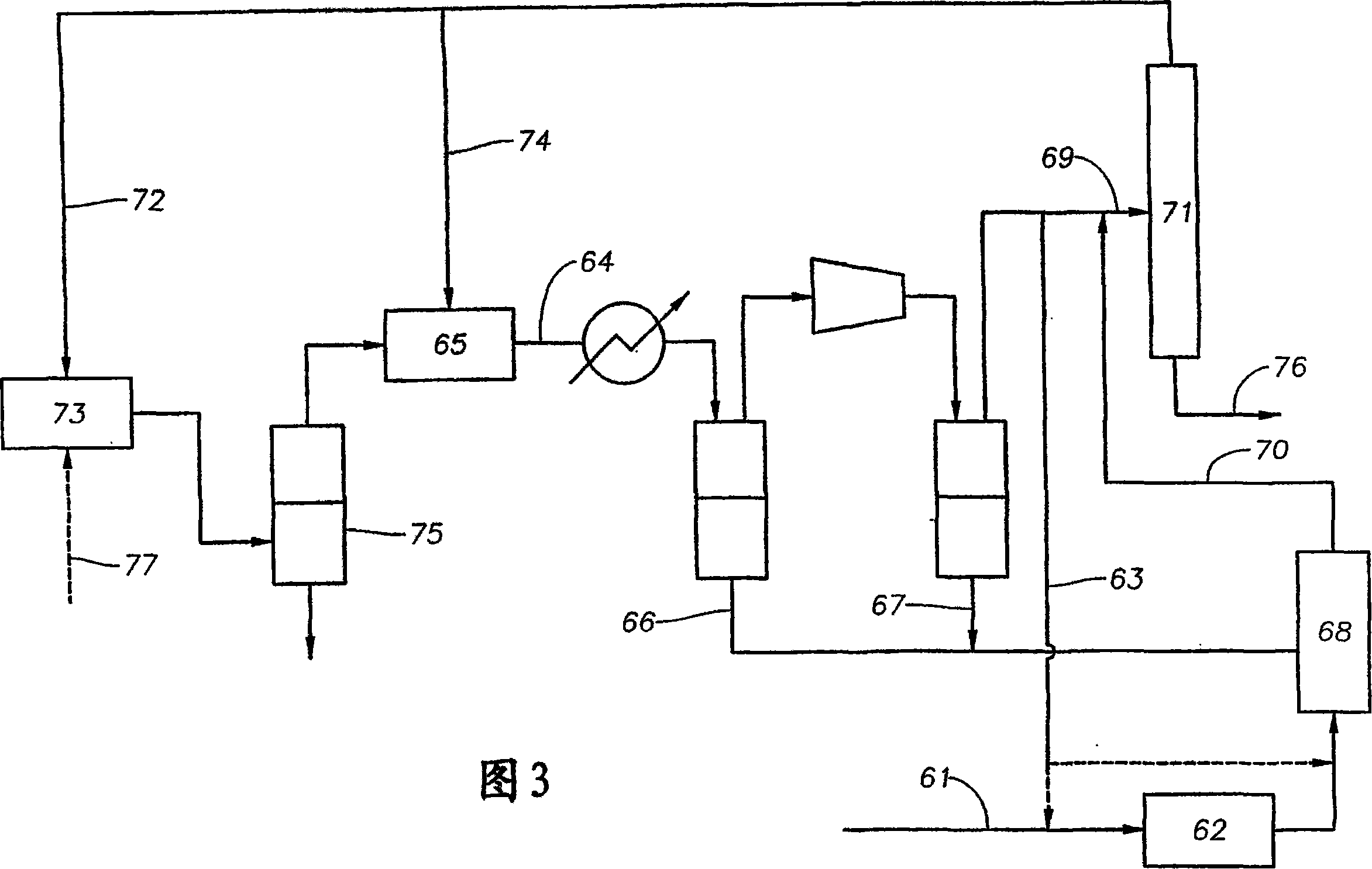
[0180] Repeat Example 1, except that the benzene generated in 100kg reductive coupling reactor 13 is mixed with 106kg methanol and 1kg H 2 combined and fed into an alkylation reactor containing a silicon-selected PZSM-5 catalyst, and at a temperature of about 501 °C and a pressure of 11 psig (177 kPa) for 3.9 hr -1 run at a WHSV of 1.8 and a hydrogen / hydrocarbon molar ratio. Separation of the alkylation effluent to remove C 8 The product stream will then be depleted in C 8 The effluent is divided into benzene, toluene and (heavy C 8 +) stream, and containing 58kg water and 10.3kg C 5 - Lightweight material flow. Again, C can be separated from the light stream 5 - components and recycled to reduction coupling reactor 13.
Embodiment 3
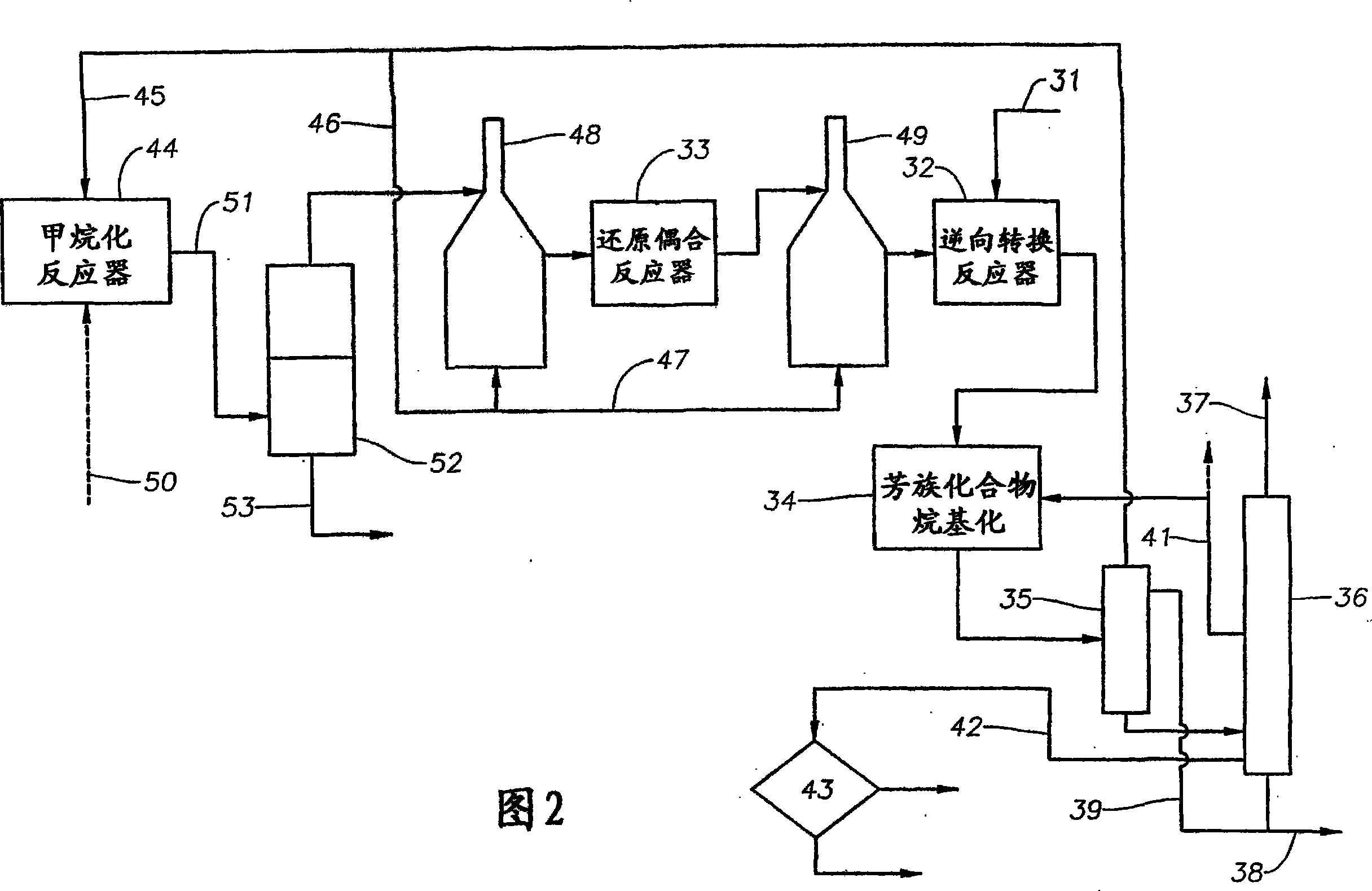
[0182] In one embodiment of the second example of the invention shown in FIG. 2 , feed 11 is a natural gas stream containing 25 wt. % carbon dioxide and is fed to reverse shift reactor 32 only. The reactor 32 contains a Ni catalyst on titania and operates at a temperature of about 900°C and a pressure of 400 psia (2,760 kPa). Alkylation reactor 13 contains supported Cr mixed with silicon-selective PZSM-5 catalyst 2 o 3 / ZnO catalyst, and operate at a temperature of about 500°C and a pressure of 400 psia (2760 kPa). The resulting aromatic product contained 38 wt% benzene, 10 wt% naphthalene, 48 wt% p-xylene and 4 wt% mixed xylenes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com