Functional membrane and process for production thereof, and electrolyte membrane for fuel cell and process for production thereof
An electrolyte membrane and fuel cell technology, which is applied to fuel cell parts, fuel cells, solid electrolyte fuel cells, etc., can solve the problems of gas barrier performance, insufficient, swelling, etc., and achieve the mentioned gas barrier performance and size The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5, Embodiment 8 and 9
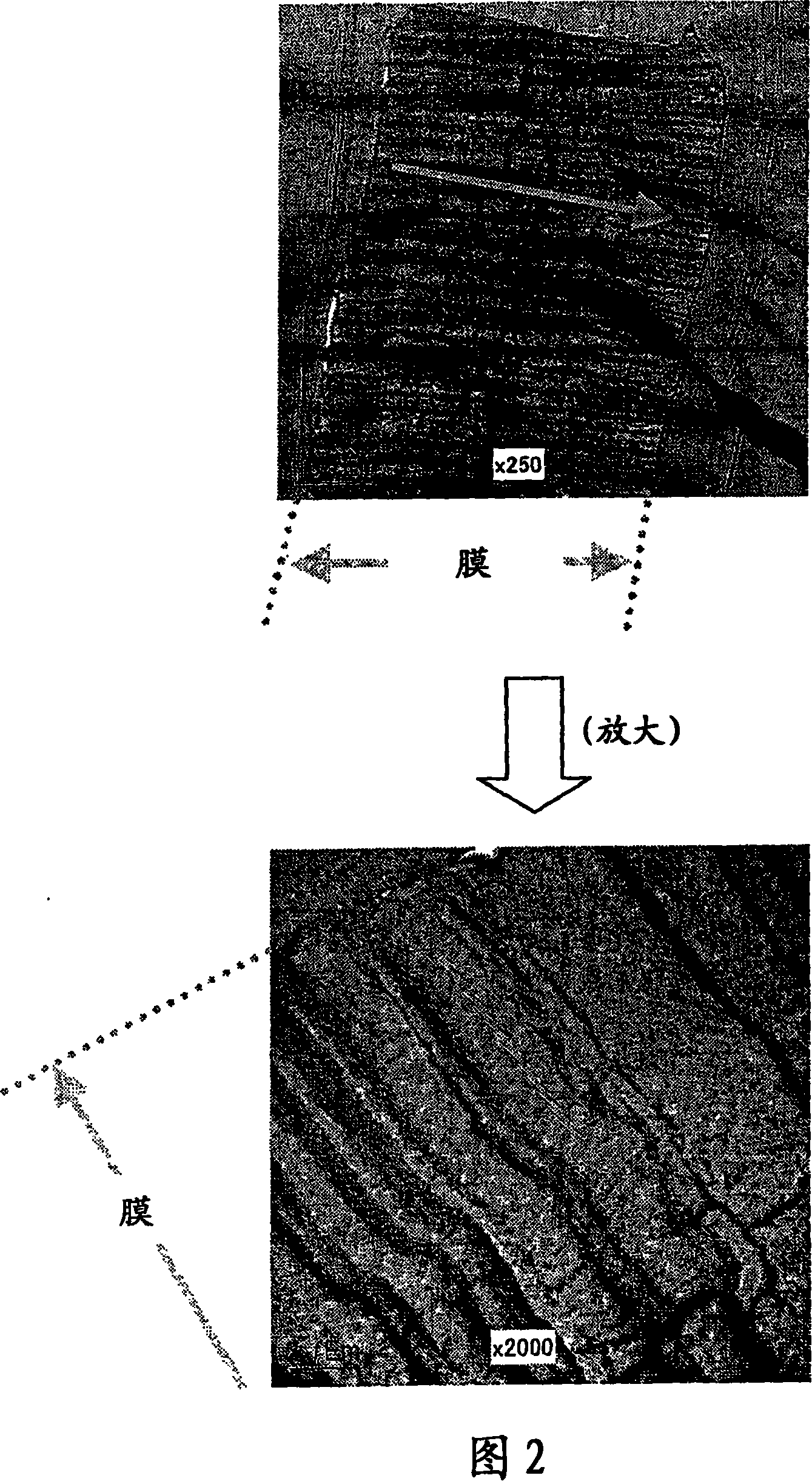
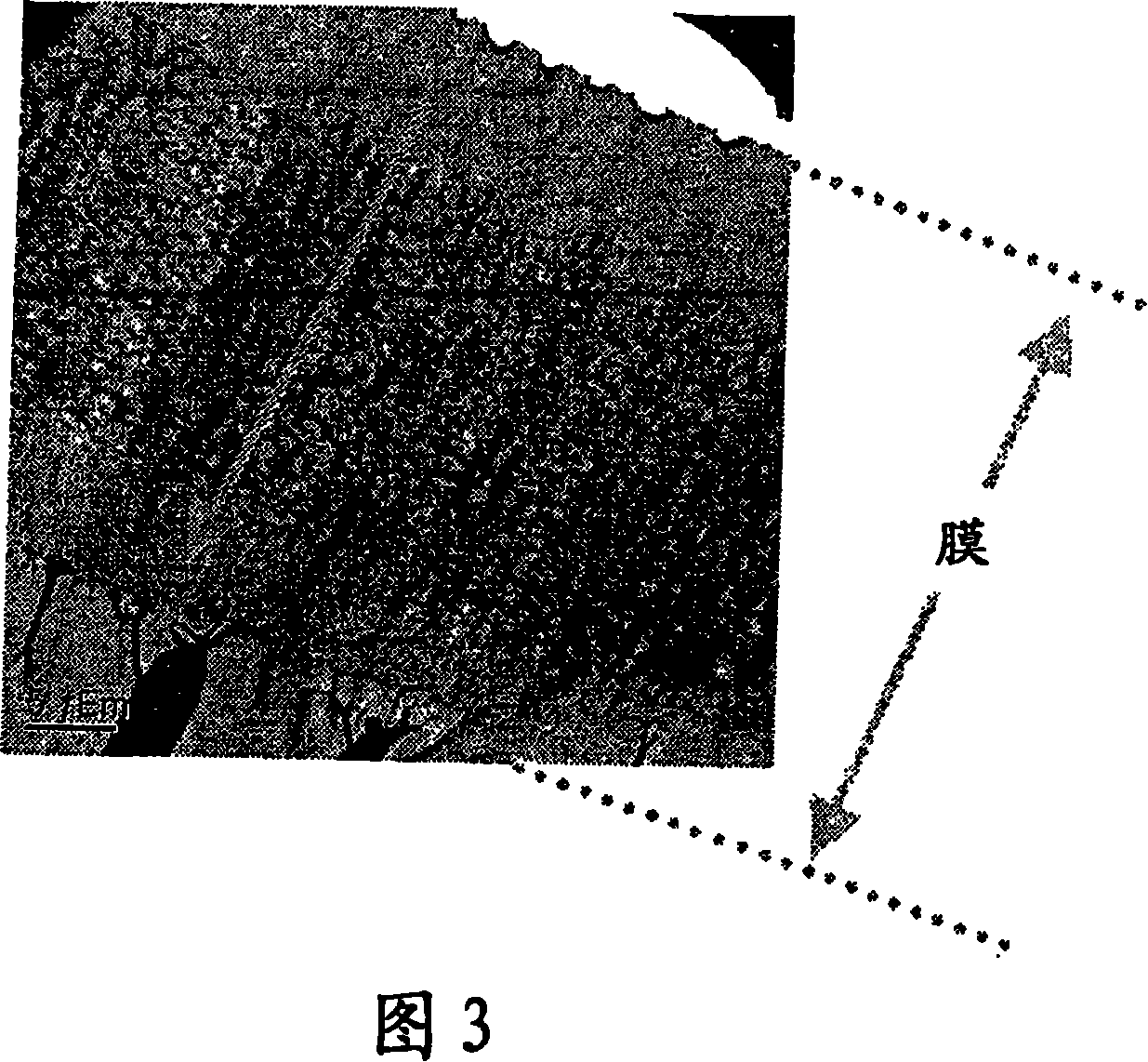
[0091] As the base material (sample film), a polyvinylidene fluoride (hereinafter, abbreviated as PVDF) film was used. The investigated monomers and corresponding compositions are shown in Table 1 provided below.
[0092] The procedure for preparing each sample is as follows.
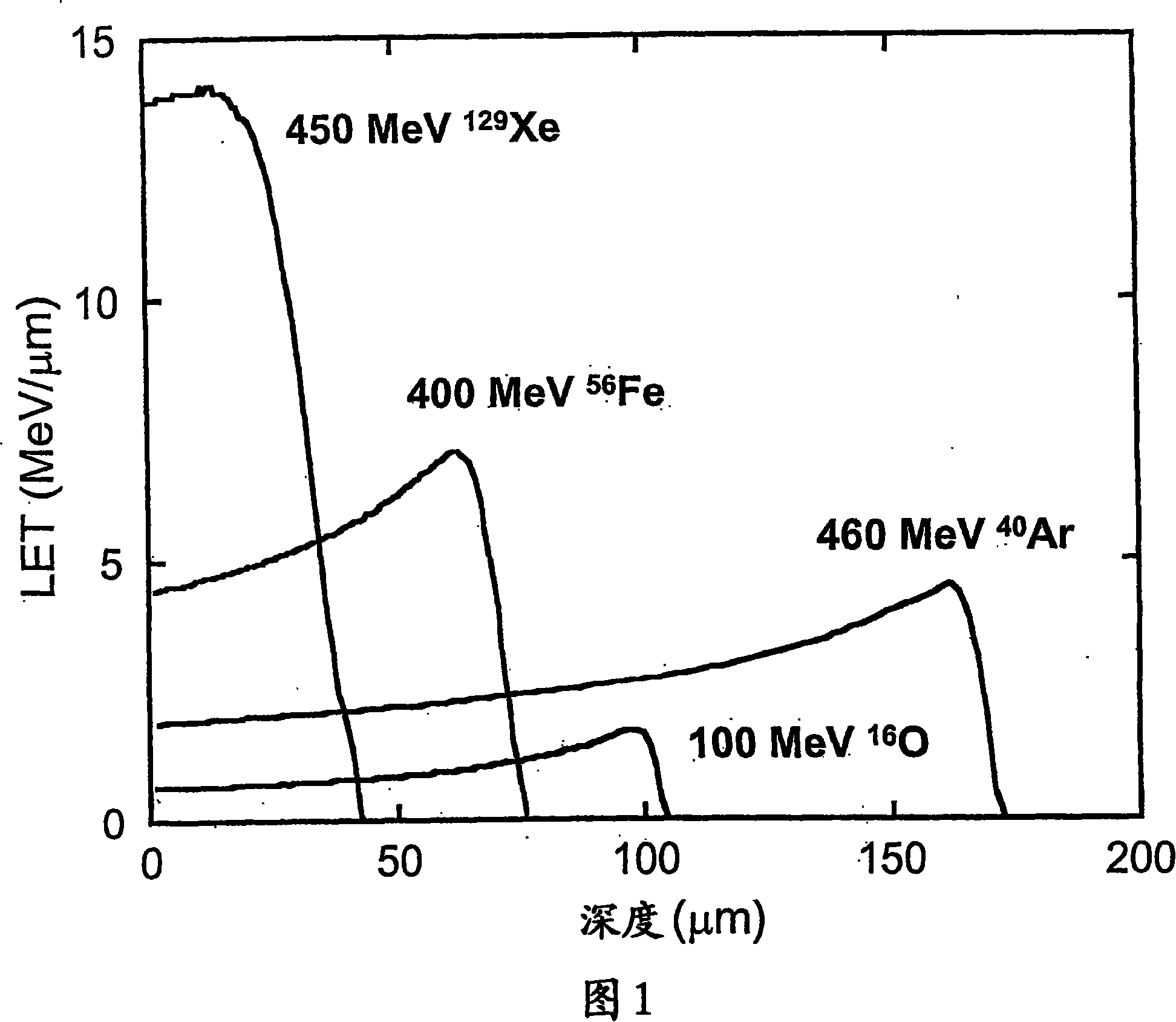
[0093] (1) The sample film is irradiated with Xe heavy ions or Au heavy ions accelerated by a cyclotron. The ion irradiation density (energy density) is controlled according to the irradiation time.
[0094] (2) The sample was taken out from the irradiation chamber into the air and immersed in the monomer solution shown in Table 1.
[0095] (3) The monomer solution was heated to 60° C. and polymerization was performed for a polymerization time of 24 hours.
[0096] (4) The sample was taken out and immersed in toluene, and the solution was heated to 60° C. to remove the homopolymer.
[0097] (5) Dry the sample in a vacuum drying oven.
[0098] (6) The sample was immersed in a 0.2 mol / l 1,2-dichloroe...
Embodiment 6 and 7
[0102] For the substrate (sample film), a PVDF film was used. The investigated monomers and corresponding compositions are shown in Table 1 provided below.
[0103] (1) The sample film is irradiated with Xe heavy ions or Au heavy ions accelerated by a cyclotron. The ion irradiation density (energy density) was controlled according to the irradiation time (the same as in Examples 1-5).
[0104] (2) The sample was taken out from the irradiation chamber into the air, etched under the alkali treatment conditions shown in Table 1, and then immersed in the monomer solution shown in Table 1 below.
[0105] (3) The monomer solution was heated to 60° C. and polymerization was performed for a polymerization time of 24 hours.
[0106] (4) to (8) are the same as in Examples 1-5.
Embodiment 10
[0108] For the substrate (sample film), a polyvinylidene fluoride (PVDF) film was used, and PVDF was subjected to γ-ray irradiation at the dose shown in Table 1 to prepare crosslinked PVDF. The investigated monomers and corresponding compositions are shown in Table 1 provided below.
[0109] (1) to (8) are the same as in Examples 1-5.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com