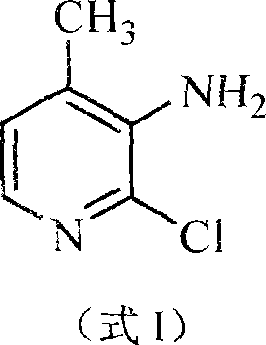
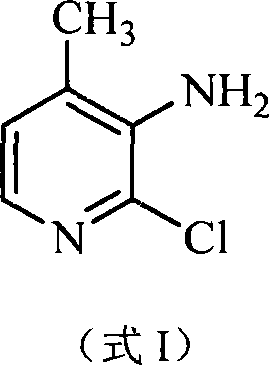
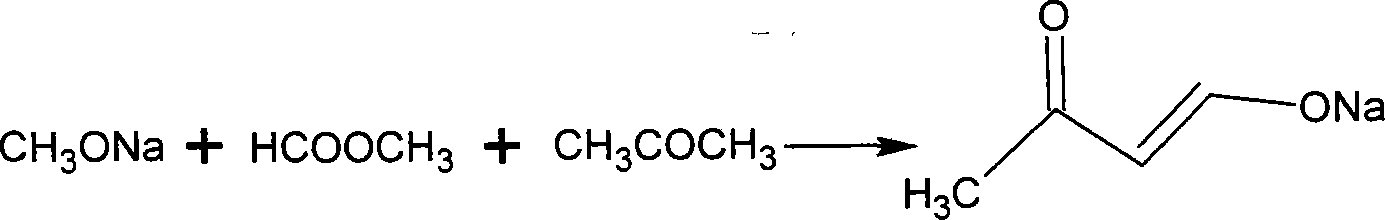Preparation method of 2-chlorin-3-amido-4-methyl pyridine
A kind of methylpyridine and amino technology, which is applied in the synthesis field of 2-chloro-3-amino-4-methylpyridine, can solve the problems such as unobtainable raw materials, high equipment requirements, cumbersome steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Preparation of sodium but-3-en-2-one-4-alcohol
[0028]
[0029] Put 40mL of methanol into a 500mL four-neck flask, cool it down, then add 0.2mol 27% sodium methoxide, and stir to make it completely dissolve. Then, a mixed solution of 0.34 mol of methyl formate and 13.0 g (0.2 mol) of acetone was added dropwise at 25°C. Afterwards, it was kept in a water bath at 40° C. for 3 hours to obtain 20.0 g of sodium but-3-en-2-one-4-olate with a purity of 98.8% and a yield of 92.6%.
[0030] (2) Preparation of 4,4-dimethoxybutan-2-one
[0031]
[0032] In the four-necked flask connected with a thermometer, an air inlet pipe and an exhaust pipe, first add 50mL of methanol, and then add concentrated sulfuric acid dropwise to make the pH of the solution 0-3. Then, 21.6 g of sodium but-3-en-2-one-4-oxide was added dropwise under ice cooling. After dripping, it was transferred to a water bath at 85°C and refluxed for 3h. Afterwards, neutralize with 20% NaOH solution und...
Embodiment 2
[0048] (1) Preparation of sodium but-3-en-2-one-4-alcohol
[0049] Synthesized according to the method and conditions of Example 1 (1), only methyl formate was replaced by ethyl formate to obtain but-3-en-2-one-4-alcohol sodium with a purity of 99.3% and a yield of 92.5%.
[0050] (2) Preparation of 4,4-dimethoxybutan-2-one
[0051] Synthesize as in Example 1 (2) method and conditions.
[0052] (3) Condensate 4,4-dicyano-3-methyl-3-butene dimethyl acetal and 1,1-dicyano-4-methoxy-2-methyl-1,3- Preparation of butadiene
[0053] Synthesize as in Example 1 (3) method and conditions.
[0054] (4) Preparation of 3-cyano-4-methylpyridin-2-one
[0055] Synthesize as in Example 1 (4) method and conditions.
[0056] (5) Preparation of 2-chloro-3-cyano-4-methylpyridine
[0057] Synthesize as in Example 1 (5) method and conditions.
[0058] (6) Preparation of 2-chloro-4-methylpyridine-3-carboxamide
[0059] Synthesize as in Example 1 (6) method and conditions.
[0060] (7) Prepa...
Embodiment 3
[0063] (1) Preparation of sodium but-3-en-2-one-4-alcohol
[0064] Synthesized according to the method and conditions of Example 1 (1), only methyl formate was replaced by propyl formate to obtain but-3-en-2-one-4-alcohol sodium with a purity of 98.3% and a yield of 91.2%.
[0065] (2) Preparation of 4,4-dimethoxybutan-2-one
[0066] Synthesize as in Example 1 (2) method and conditions.
[0067] (3) Condensate 4,4-dicyano-3-methyl-3-butene dimethyl acetal and 1,1-dicyano-4-methoxy-2-methyl-1,3- Preparation of butadiene
[0068] Synthesize as in Example 1 (3) method and conditions, only malononitrile is replaced by cyanoacetamide to obtain a condensate.
[0069] (4) Preparation of 3-cyano-4-methylpyridin-2-one
[0070] Synthesize as in Example 1 (4) method and conditions.
[0071] (5) Preparation of 2-chloro-3-cyano-4-methylpyridine
[0072] Synthesize as in Example 1 (5) method and conditions.
[0073] (6) Preparation of 2-chloro-4-methylpyridine-3-carboxamide
[0074]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com