Method for synthesizing N,N,N-trimethyl chitosan sulfate methyl ammonium
A technology of trimethyl chitosan and ammonium methyl sulfate, which is applied in the field of organic synthesis, can solve the problems of high cost and large dosage, and achieve the effects of strong antibacterial property, wide application field and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
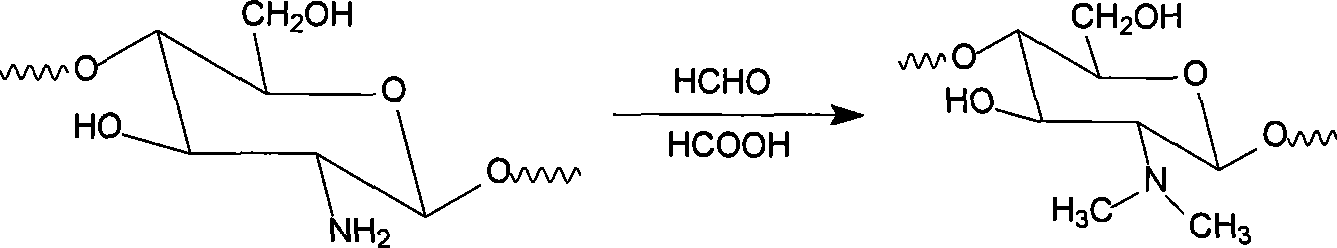
[0028] (1) Preparation of intermediate N,N-dimethyl chitosan
[0029] Weigh 4g (0.025mol) of chitosan in a three-necked flask, add 5mL of formic acid (0.088mol) and 200mL of water, heat to 55°C, stir until the chitosan dissolves, add 6mL of 36% formaldehyde (0.078mol), 55 The reaction was stirred at °C for 6 h. The reaction solution was cooled to room temperature, 10% NaOH solution was gradually added, the pH value was adjusted to 11, and the chitosan was completely precipitated by standing for 20 minutes, suction filtered, washed with deionized water until neutral, and dried below 70 °C to obtain a powdery light 4.1 g of yellow product.
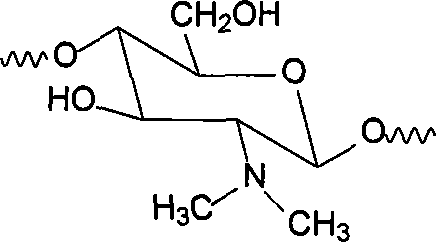
[0030] The product is infrared, 13 Characterized by C-NMR, thermogravimetric analysis and differential scanning calorimetry analysis, it was identified as N,N-dimethyl chitosan, and its structural unit is:
[0031]
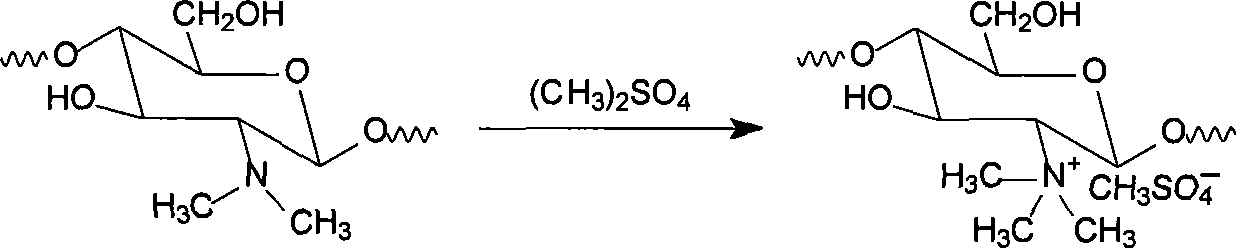
[0032] (2) Preparation of target product N,N,N-trimethyl chitosan ammonium methyl sulfate (TMCS)
[0033] Weigh 4g of N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com