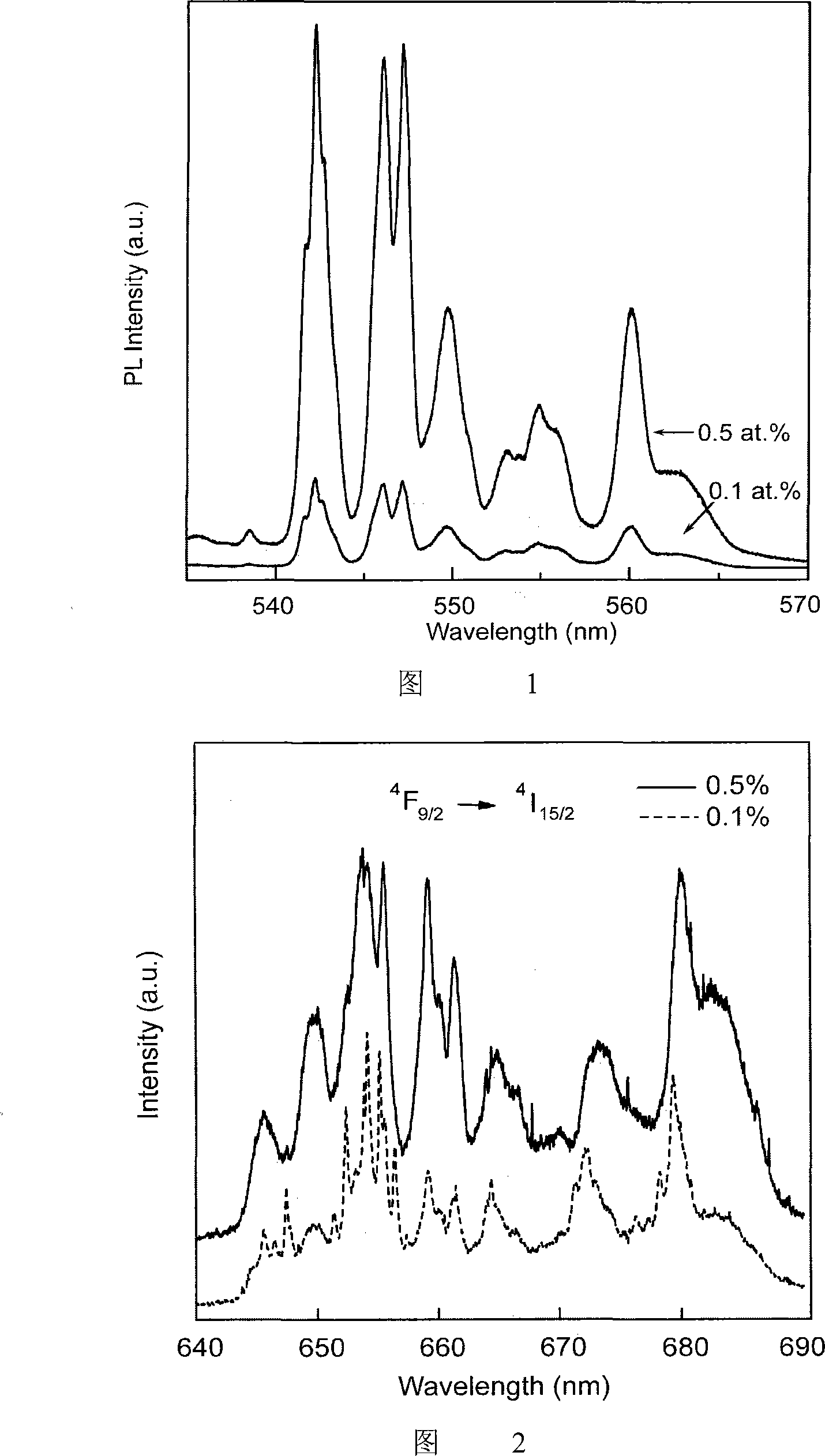
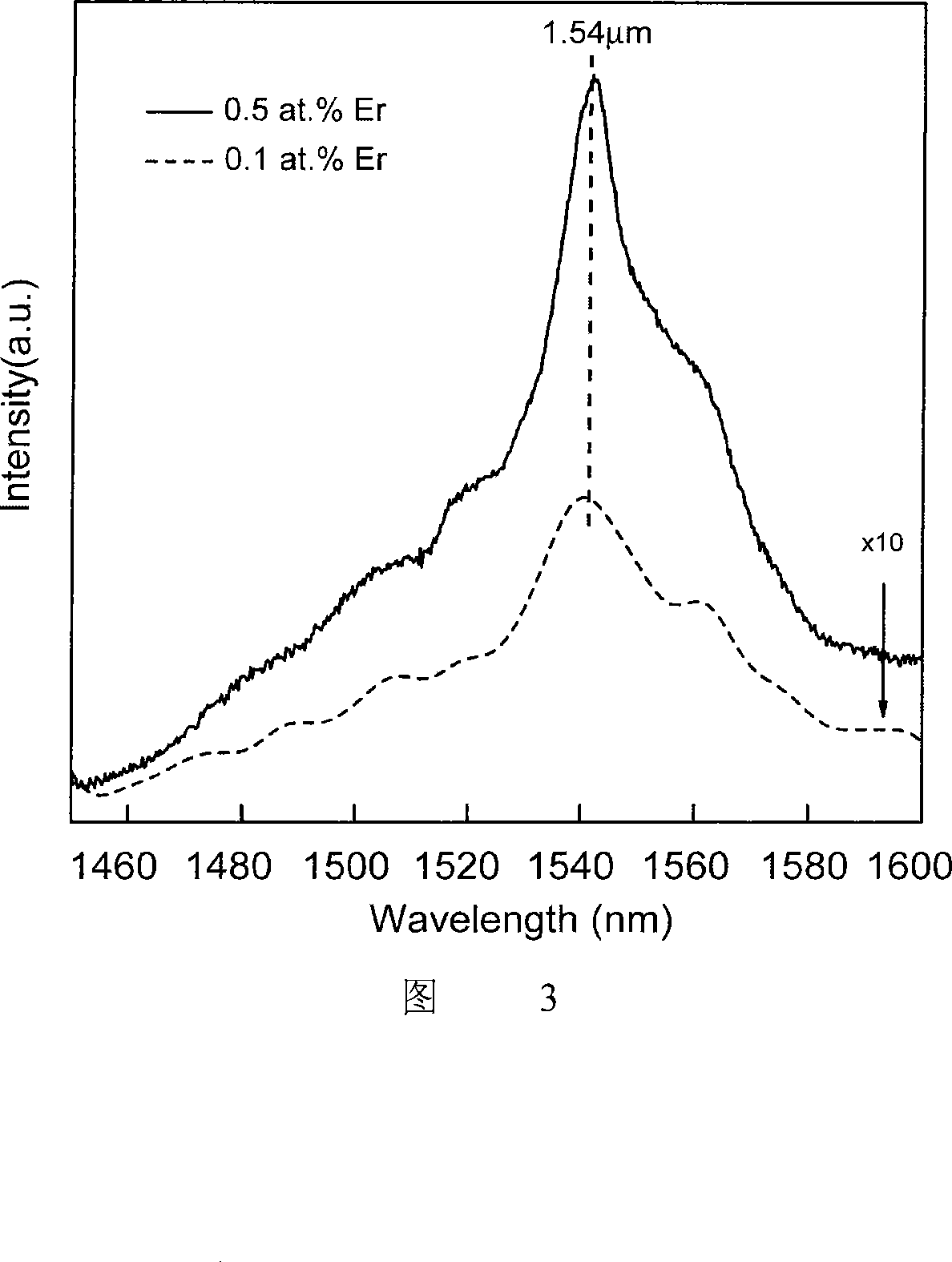
Visible and infrared luminescent C12A7 powder and preparation method thereof
A technology of infrared luminescence and dodecacalcium, which is applied in the field of materials and can solve problems affecting the luminous efficiency of rare earths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. get 7.878g (0.02100mol) purity to be 99.99% Al (NO 3 ) 3 powder and 1.998 g (0.01800 mol) of 99.95% pure CaCl 2 The raw materials are uniformly mixed in the water phase at the atomic level according to the chemical dosage ratio of 14:12;
[0021] 2. Add 0.04800g (0.000105mol) of Er(NO) with a purity of 99.99% 3 ) 3 The raw materials are doped according to the atomic molar ratio of erbium to aluminum at 0.5%, and the precipitant NH is added after uniform mixing 3 .H 2 O, to adjust the distance between cations. Slowly pour the mixed solution into the excess NH 3 .H 2 O in a beaker to ensure that each cation can be uniformly precipitated according to a certain ratio without causing phase separation, and 0.5% erbium-doped heptaaluminate dodecacalcium (12CaO 7Al 2 o 3 ) gel precursor.
[0022] 3. The obtained erbium-doped calcium heptaaluminate (12CaO·7Al 2 o 3 ) The gel precursor is dried and dehydrated at a temperature of 100°C;
[0023] 4. Put it in a high...
Embodiment 2
[0026] 1. get 7.787g (0.02100mol) purity to be 99.99% Al (NO 3 ) 3 powder and 1.998 g (0.01800 mol) of 99.95% pure CaCl 2 The raw materials are uniformly mixed in the water phase at the atomic level according to the chemical dosage ratio of 14:12;
[0027] 2. Add 0.009688g (0.000021mol) of 99.99% Er(NO 3 ) 3 The raw materials are doped according to the atomic molar ratio of erbium to aluminum at 0.1%, and the precipitant NH is added after uniform mixing 3 .H 2 O, to adjust the distance between cations. Slowly pour the mixed solution into the excess NH 3 .H 2 O in a beaker to ensure that each cation can be uniformly precipitated according to a certain ratio without causing phase separation, and 0.1% erbium-doped heptaaluminate dodecacalcium (12CaO 7Al 2 o 3 ) gel precursor.
[0028] 3. The obtained 0.1% erbium doped calcium heptaaluminate (12CaO·7Al 2 o 3 ) The gel precursor is dried and dehydrated at a temperature of 90°C;
[0029] 4. Put it in a high-temperature ...
Embodiment 3
[0032] Steps 1 and 2 are the same as in the embodiment, the drying and water removal time in step 3 is 120°C, the sintering temperature in step 4 is 1250°C, and the time is 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com