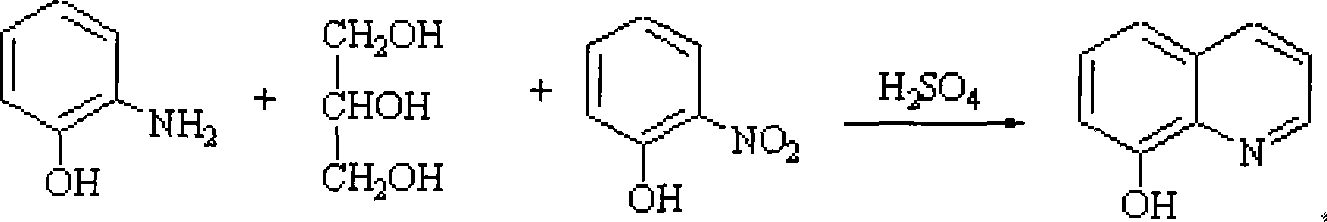
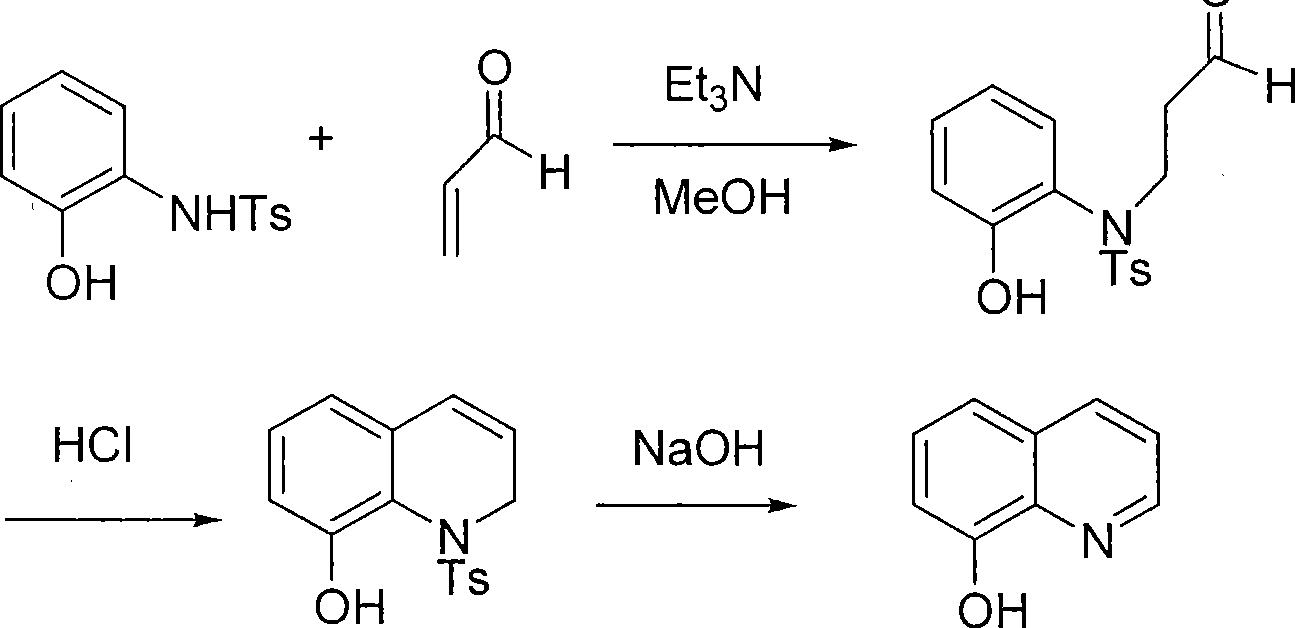
Production technique of 8-hydroxyquinoline
A production process, the technology of hydroxyquinoline, applied in the direction of organic chemistry, etc., can solve the problems of low reaction yield, limited source, expensive price, etc., and achieve simple feeding and post-processing, safe and mild reaction conditions, and low equipment investment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1) Mix 1 mole of 2-N-p-toluenesulfonamidophenol, 0.02 mole of triethylamine, and 1000 ml of methanol and cool, then add 1.0 mole of acrolein dropwise at -20°C for 1 hour; , continue to stir and react at -20°C for 1 hour; then add hydrochloric acid, raise the temperature to 20°C and stir for 1 hour to generate N-p-toluenesulfonyl-8-hydroxyhydroquinoline intermediate;
[0025] 2) Add solid sodium hydroxide to the above solution, heat up and reflux for 1 hour, cool down, and then adjust the pH to 7.5 with hydrochloric acid;
[0026] 3) Add water to the above solution, heat up and carry out steam distillation to obtain 0.75 mole of high-purity 8-hydroxyquinoline; based on 2-N-p-toluenesulfonamidophenol, the molar yield is 75%.
Embodiment 2
[0028] 1) Mix 1 mole of 2-N-p-toluenesulfonamidophenol, 0.2 moles of triethylamine, and 1000 ml of methanol and cool, then add 1.6 moles of acrolein dropwise at -5°C for 10 hours; , continue to stir and react at -5°C for 5 hours; then add hydrochloric acid, heat up to 50°C and stir for 5 hours to generate N-p-toluenesulfonyl-8-hydroxyhydroquinoline intermediate;
[0029] 2) Add solid sodium hydroxide to the above solution, heat up and reflux for 5 hours, then cool down, then adjust the pH to 8.5 with hydrochloric acid;
[0030] 3) Add water to the above solution, heat up and carry out steam distillation to obtain 0.80 mole of high-purity 8-hydroxyquinoline; based on 2-N-p-toluenesulfonamidophenol, the molar yield is 80%.
Embodiment 3
[0032] 1) Mix 1 mole of 2-N-p-toluenesulfonamidophenol, 0.05 mole of triethylamine, and 1000 ml of methanol and cool, then add 1.2 moles of acrolein dropwise at -10°C for 5 hours; , continue to stir and react at -20°C for 3 hours; then add hydrochloric acid, heat up to 30°C and stir for 3 hours to generate N-p-toluenesulfonyl-8-hydroxyhydroquinoline intermediate;
[0033] 2) Add solid sodium hydroxide to the above solution, heat up and reflux for 3 hours, cool down, and then adjust the pH to 8.0 with hydrochloric acid;
[0034] 3) Add water to the above solution, heat up and carry out steam distillation to obtain 0.85 mole of high-purity 8-hydroxyquinoline; based on 2-N-p-toluenesulfonamidophenol, the molar yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com