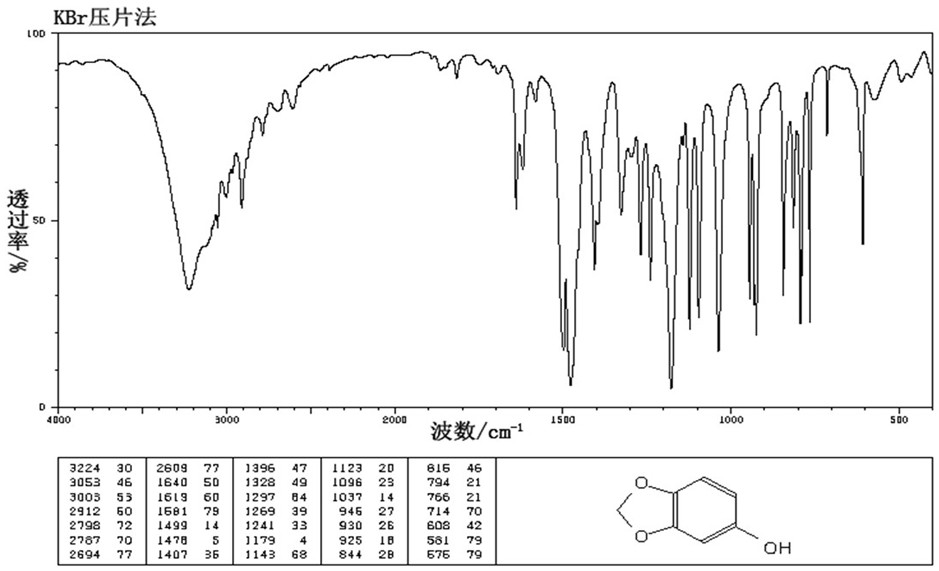
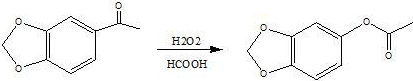
A kind of preparation method of 3,4-methylenedioxyphenol
A technology of dioxyphenol and dioxyacetophenone, which is applied in the field of preparation of heterocyclic compounds, can solve the problems of low yield and high cost of raw materials, and achieves the effects of high yield, simple operation and broad application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put 100ml of toluene, 36g of formic acid, and 36g of 3,4-methylenedioxyacetophenone into a four-necked reaction flask, stir and heat to 30-40°C, and keep for 30 minutes; slowly add 36g of 50% hydrogen peroxide dropwise, and the dropping time 12 hours.
[0038] After the dropwise addition, keep stirring for 1 hour, then let stand for 30 minutes, remove the lower layer of acid water, wash the toluene layer with 50ml of water, remove the lower layer of water, desolvate the toluene layer under reduced pressure, remove all the toluene, and use the residue for future use.
[0039] Add 140ml of water and 60g of 30% liquid caustic soda into the four-necked reaction flask, add the raffinate obtained in the previous step, and stir and react at 35-40°C for 4 hours. After the reaction is completed, add 10% hydrochloric acid to adjust the pH value to 6-6.5 , and stirred for 1 hour.
[0040] Stand still for 30 minutes, remove the lower water layer, collect the oil layer, rectify the...
Embodiment 2
[0045] Put 100ml of toluene, 72g of formic acid, and 36g of 3,4-methylenedioxyacetophenone into a four-necked reaction flask, stir and heat to 30-40°C, and keep it for 30 minutes; slowly add 36g of 50% hydrogen peroxide dropwise, and the dropping time 12 hours. After the dropwise addition, keep stirring for 1 hour, let stand for 30 minutes, remove the lower layer of acid water, wash the toluene layer with 50ml of water, remove the lower layer of water, desolvate the toluene layer under reduced pressure, remove all the toluene, and use the residue for later use.
[0046] Add 140ml of water and 60g of 30% liquid caustic soda into the four-necked reaction flask, add the raffinate in Example 1, stir and react at 35-40°C for 4 hours, when the reaction is over, add 10% hydrochloric acid to adjust the pH to 6~6.5, and stir React for 1 hour. After standing still for 30 minutes, the lower water layer was removed, and the oil layer was collected. The oil layer was rectified under high ...
Embodiment 3
[0048] Put 200ml of toluene, 144g of formic acid, and 72g of 3,4-methylenedioxyacetophenone into a four-necked reaction flask, stir and heat to 30-40°C, and keep for 30 minutes; slowly add 72g of 50% hydrogen peroxide dropwise, and the dropping time 12 hours. After the dropwise addition, keep stirring for 1 hour, let stand for 30 minutes, remove the lower layer of acid water, wash the toluene layer with 100ml of water, remove the lower layer of water, desolvate the toluene layer under reduced pressure, remove all the toluene, and use the residue for future use.
[0049] Add 280ml of water and 120g of 30% liquid caustic soda into the four-necked reaction flask, add the raffinate in Example 3, stir and react at 35-40°C for 4 hours, after the reaction is completed, add 10% hydrochloric acid to adjust the pH to 6~6.5, and stir React for 1 hour. After standing still for 30 minutes, the lower water layer was removed, and the oil layer was collected. The oil layer was rectified unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com