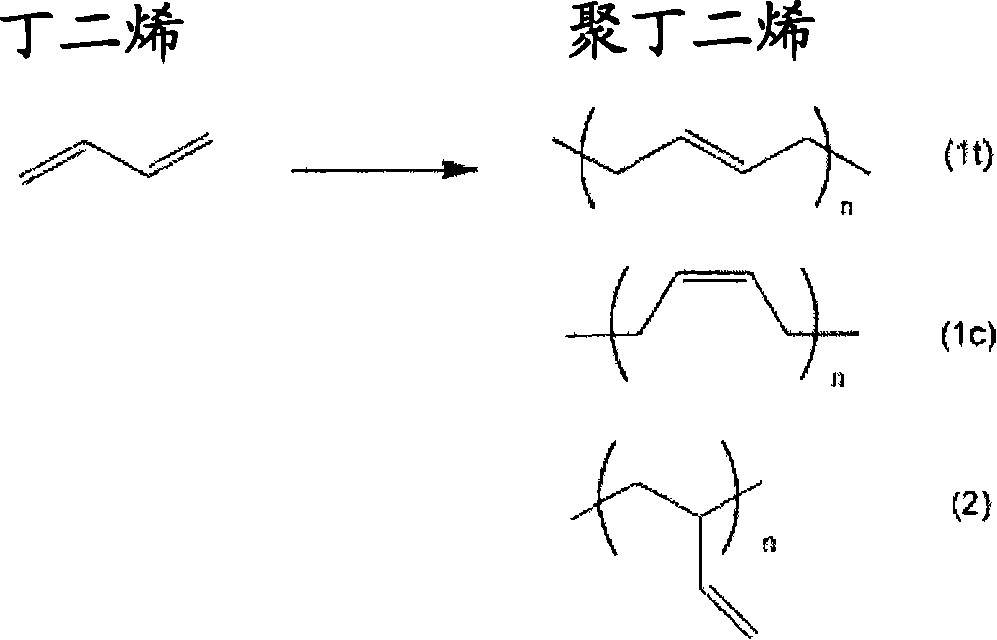
Carboxyl group-containing polyurethane, heat-curable resin composition and uses thereof
A technology of polyurethane resin and thermosetting resin, which is applied in the field of thermosetting resin composition, which can solve the problems of poor substrate adhesion, etc., and achieve the effects of excellent soldering heat resistance, excellent long-term reliability, and low twist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment a1
[0207] To the reaction vessel equipped with a stirrer, a thermometer and a condenser, 1172 g of polymer polyol polybutadiene (G-1000 manufactured by NIPPON SODA CO., LTD.) having 1,2-repeating units, 184.5 g of carboxyl group-containing The dihydroxy compound 2,2-dimethylolbutyric acid (manufactured by Nippon Kasei Chemical Co., Ltd.) and 1744 g of diethylene glycol ethyl ether acetate (manufactured by DAICEL CHEMICAL INDUSTRIES, LTD.) as a solvent. The material was dissolved at 90°C. The temperature of the reaction liquid was lowered to 70° C., and 125 g (0.48 mol) of polyisocyanate DESMODUR W (manufactured by Sumika Bayer Urethane Co., Ltd.) was added dropwise with a dropping funnel over 30 minutes. After the dropwise addition was complete, the reaction was allowed to proceed at 80°C for 3 hours, at 90°C for 3 hours, and at 100°C for 3 hours. When it was confirmed that the isocyanate substantially disappeared, 4.4 g (0.06 mol) of isobutanol (manufactured by Wako Pure Chemic...
Embodiment a2
[0210] 73.9 g of polymer polyol polybutadiene (G-1000 manufactured by NIPPON SODA CO., LTD.) having 1,2-repeating units were added to a reaction vessel equipped with a stirrer, a thermometer, and a condenser, 12.0 g containing 2,2-dimethylolpropionic acid (manufactured by Nippon Kasei Chemical Co., Ltd.), a dihydroxy compound of carboxyl group, and 125.1 g of diethylene glycol ethyl ether acetate (manufactured by DAICELCHEMICAL INDUSTRIES, LTD.) as a solvent. The material was dissolved at 90°C. The temperature of the reaction liquid was lowered to 70° C., and 36.0 g (0.137 mol) of polyisocyanate DESMODUR W (manufactured by Sumika Bayer Urethane Co., Ltd.) was added dropwise with a dropping funnel over 30 minutes. After the dropwise addition was complete, the reaction was carried out at 80°C for 1 hour, 90°C for 1 hour, and 100°C for 1.5 hours. When it was confirmed that the isocyanate substantially disappeared, 2.0 g (0.027 mol) of isobutanol (manufactured by Wako Pure Chemic...
Embodiment a3
[0213] 89.8 g of polymer polyol polybutadiene (G-1000 manufactured by NIPPON SODA CO., LTD.) having 1,2-repeating units were added to a reaction vessel equipped with a stirrer, a thermometer, and a condenser, 6.61 g containing 2,2-dimethylolbutanoic acid (manufactured by Nippon Kasei Chemical Co., Ltd.), a dihydroxy compound of carboxyl group, and 125.0 g of propylene glycol methyl ether acetate (manufactured by DAICELCHEMICAL INDUSTRIES, LTD.) as a solvent. The material was dissolved at 90°C. The temperature of the reaction liquid was lowered to 70° C., and 27.0 g (0.103 mol) of polyisocyanate DESMODUR W (manufactured by Sumika Bayer Urethane Co., Ltd.) was added dropwise with a dropping funnel over 30 minutes. After the dropwise addition was complete, the reaction was allowed to proceed at 80°C for 1 hour, at 90°C for 1 hour, and at 100°C for 1.5 hours. When it was confirmed that the isocyanate substantially disappeared, 1.5 g (0.021 mol) of isobutanol (manufactured by Wako...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com